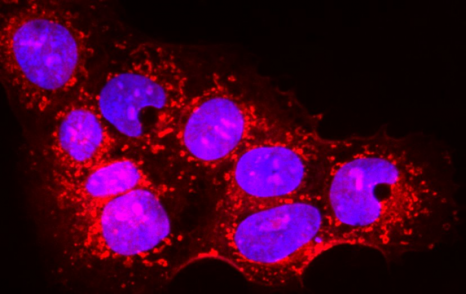
The dysfunction of the cytoskeleton, a constituent element of the cell, is often associated with pathologies such as the onset of metastases. For this reason, it is a target of interest in numerous therapies. Teams from CNRS, the Université de Strasbourg and Inserm, led by Daniel Riveline1, Jean-Marie Lehn2 and Marie-France Carlier3, have synthesized molecules capable of causing rapid growth of actin networks, one of the components of the cytoskeleton. This is a breakthrough because, until now, only molecules that stabilize or destroy the cytoskeleton of actin have been available. These compounds with novel properties, whose action has been elucidated both in vitro and in vivo, provide a new tool in pharmacology. This work was published in the journal Nature Communications on 29 July 2013.
The cytoskeleton is mainly composed of actin filaments and microtubules. Made of polymers in dynamic assembly and constantly constructing and deconstructing itself, it affects numerous cellular processes such as intracellular movement, division and transport. It is involved in key steps of embryogenesis and other processes essential to life. Consequently, its malfunctioning can lead to serious pathologies. For example, the onset of certain metastases is revealed by an increased activity of the cytoskeleton. Identifying new molecules that target the cytoskeleton thus represents a major challenge.
Until now, the molecules known and used in pharmacology had the effect of stabilizing or destroying the cytoskeleton of actin. Actin allows vital actions to be performed by assembling and disassembling itself spontaneously, continually and rapidly in the form of filaments that organize themselves and form networks of parallel bundles or intertwined meshes (known as lamellar networks). Derived from supramolecular chemistry[4], the new compounds synthesized by the researchers have original properties: within several minutes, they bring about the growth of lamellar networks of actin filaments. This is the first time that a pharmacological tool induces growth of the actin network — something that living organisms do all the time. In this way, the researchers have shown that the action of these compounds is specific in vivo (on cells). In addition, they have identified the growth mechanism of the actin network by comparative in vivo and in vitro studies in order to ensure the validity of the process.
For cellular or molecular biology, this tool proposes a new mode of possible action on the cytoskeleton and thus opens new research perspectives for deciphering the living world. This finding could lead to the development of new compounds, derived from the same chemistry, and potential candidates for new therapies targeting the cytoskeleton.
[1] Institut de Science et d’Ingénierie Supramoléculaires (CNRS/Université de Strasbourg) and Institut de Génétique et de Biologie Moléculaire et Cellulaire (CNRS/Université de Strasbourg/Inserm).
[2] Institut de Science et d’Ingénierie Supramoléculaires (CNRS/Université de Strasbourg).
[3] Laboratoire d’Enzymologie et Biochimie Structurales of CNRS.
[4] Supramolecular chemistry, the science of self-assembly and self-organization at the molecular scale, focuses on chemical entities resulting from the interactions between molecular objects.