On hearing the word ‘memory’, the brain normally springs to mind… or perhaps our immune system, which memorizes data to react more effectively should bacteria infect us a second time. But who would ever have imagined that our endocrine glands also ‘remember’ certain data? A group of Inserm and CNRS researchers, supervised by Patrice Mollard at the Institute of Functional Genomics (1) (Montpellier), have recently demonstrated that, in mice, hypophyseal endocrine cells used to control lactation are organised into a network (like those in the brain) during the first feeding phase. This network is then conserved, or “memorized”, for even greater functional efficiency during the feeding of a second litter. It is the first time that an experience-dependent ‘memory’ has been revealed in the endocrine system. The research is the subject of an article published in the Nature Communications review dated 3 January 2012.
The plasticity of biological systems makes it possible for organisms to dynamically modify their physiology to adapt to existing environmental conditions. In terms of cells, this process is usually associated with the immune system; with regard to tissue, this process was characterized in the brain many years ago and is at the heart of intense neurobiological research.
Besides these two systems used to memorize data over the long-term, there was no evidence suggesting other cells could operate in a similar manner.
The pituitary gland represents an ideal model to check this hypothesis since it has distinct populations of endocrine cells, organised into networks, which secrete different hormones to control a multitude of physiological functions.
Patrice Mollard’s team in Montpellier worked in conjunction with Paul Le Tissier’s team in London (NIMR-MRC) (2) to determine whether the endocrine cell networks had any memorization capacities. The cells that secrete prolactin (lactation hormone) were used as a model. Prolactin secretion commands a range of responses that are crucial for the feeding of baby mice, including milk production.
The production of prolactin (and thus maternal milk) in mice is stimulated by lifting an inhibiting (dopaminergic) signal in the brain and by the suckling phenomenon.
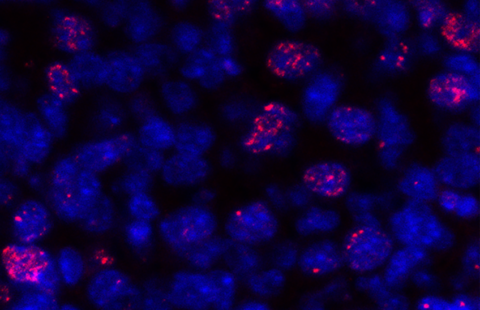
Using bi-photon calcium imaging, the researchers were able to distinguish interaction between the prolactin-producing cells, before, during and after a feeding period.
Before suckling, these cells were weakly interconnected.
During suckling, the cells responded to lactation by increasing coordinated intercellular communication, functional connectivity and tissue production.
The originality of this discovery lies in the fact that three months after weaning, the network is still in place, as if it has been memorized. “In the future”, explains Patrice Mollard, the same stimulus (suckling) will trigger a more coordinated and effective response. The network will secrete more prolactin and will again cause an increase in tissue production”.
However, the network is not created if the suckling stimulus power is reduced. Mice often have large litters (an average of eight babies per litter), if only three babies are positioned for suckling, the stimulus is too low to create the network.
It is the first time that researchers have highlighted an experience-dependent memory in a system of this kind. “It opens open a vast range of possibilities. We think that this discovery could also be applied to other endocrine systems, such as pancreatic beta cells and endocrine cells in the gastrointestinal tract”, conclude the authors.
Notes
(1) (CNRS / Inserm / Universités de Montpellier 1 & 2)
(2) MRC National Institute for Medical Research