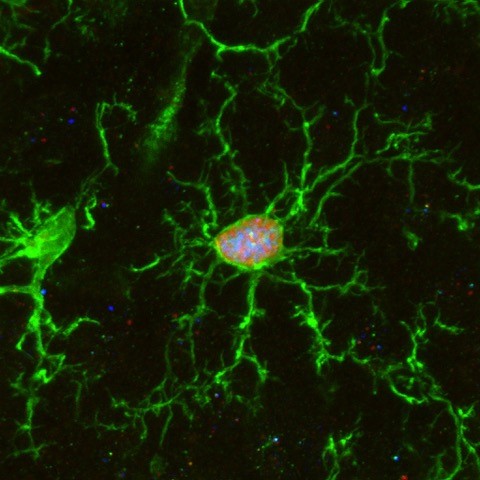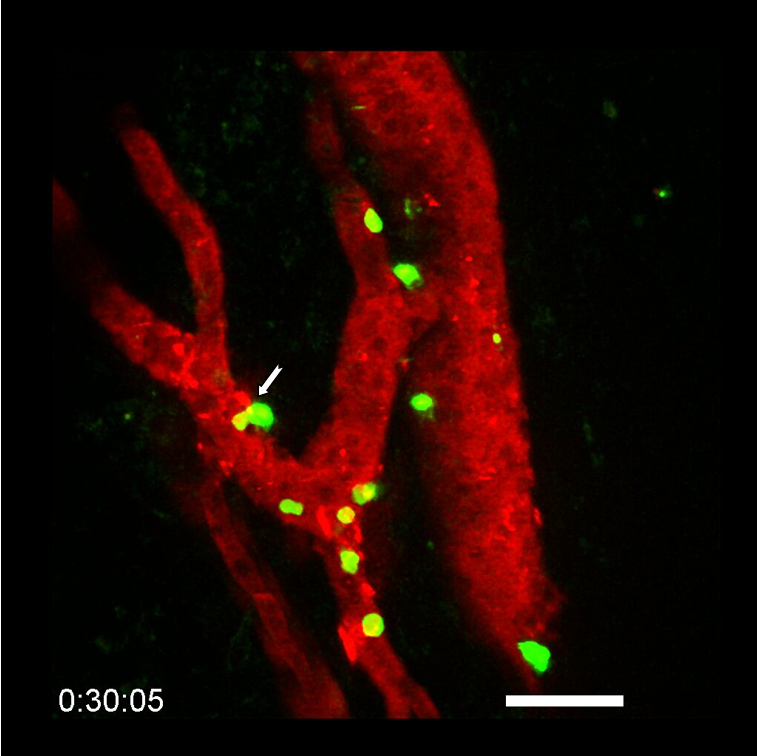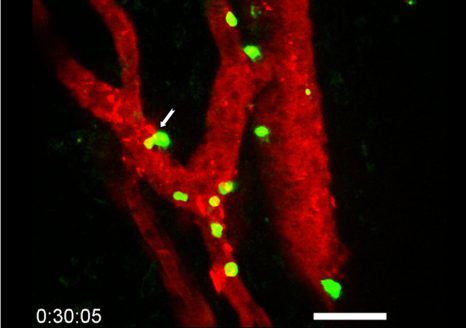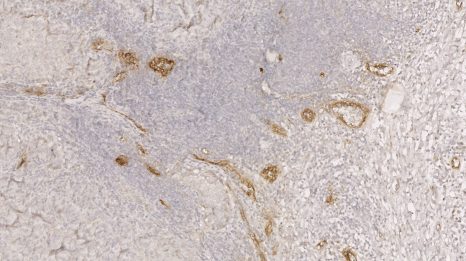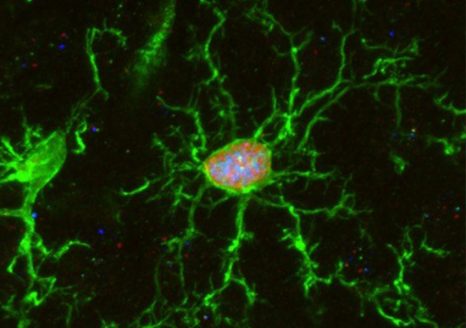
Brain microglia (green) initiating expression of cell division marker (red), but unable divide due to co-expression of a senescence marker (blue), due to the chemotherapy treatment (busulfan). © K. Sailor/ PM Lledo, Institut Pasteur.
Annually over 50,000 bone marrow transplantations occur worldwide as a therapy for multiple cancerous and non-cancerous diseases. Yet, how this procedure gives rise to bone marrow-derived cells that engraft the brain, despite being absent in the normal brain, remains unknown. In the present study, scientists from the Institut Pasteur, the CNRS and the Paris Brain Institute (Inserm) discovered how the host’s microglia, the brain’s innate immune cells, are replaced by bone marrow-derived macrophages. The key discovery was that transplantation chemotherapy eliminated the microglia’s regenerative capacity, gradually causing the engraftment of macrophages to replace microglia, providing a potential mechanism for future cell-based therapies to treat central nervous system diseases. This finding will be published in Nature Medicine on February 21, 2022.
Progressive demyelination of the central nervous system leads to devastating neurologic symptoms and premature death. As the number of cases diagnosed through pregnancy and newborn screening is increasing, more efficient therapeutic strategies are required. Recently, the successful use of gene therapy to correct disease-causing gene mutations in bone marrow stem cells, and their subsequent autologous transplantation into patients, has been clinically used for treating white matter diseases, while eliminating the need for finding matching donors. Clinical studies showed that pre-transplantation ablation of host bone marrow stem cells using the chemotherapy agent busulfan allows for efficient engraftment and tolerance to gene-modified cells, yet the mechanism involved was largely known.
Using an animal model, neuroscientists from the Institut Pasteur (CNRS) and the Paris Brain Institute (Inserm) have demonstrated the broad impact of busulfan chemotherapy used for bone marrow transplant therapy on various cell populations of the host brain.
Microglia are tissue-resident brain immune cells that are instrumental for maintaining healthy brain physiology in normal and diseased states. These cells exhibit a robust self-renewal capacity and gradually turnover throughout life. However, in this study, Sailor and colleagues show that following pre-transplantation busulfan chemotherapy, microglia completely lose their regenerative capacity, becoming senescent, which thereby allowed the engraftment of bone marrow-derived macrophages into the brain. They also show these macrophages to become resident with similar surveillant dynamics as the microglia they replaced.

They demonstrate that busulfan administration temporarily depleted oligodendrocyte precursor cells and, importantly, caused the complete and permanent ablation of all adult neurogenesis, which may explain cognitive deficits in patients after busulfan chemotherapy, i.e. the “chemo brain”. Microglia that were eliminated because of busulfan chemotherapy, coupled with their loss of regenerative capacity, leave empty niches in the brain which are replaced by engrafted bone marrow-derived macrophages. These macrophages assumed the morphology and cellular process dynamics like normal microglia.
This study is highly relevant since for the first time it shows a mechanism dependent on microglia senescence, with the loss of their regenerative capacity, to explain how macrophages are permissively engrafted into the brain after bone marrow transplantation.
Furthermore, this work shows multiple off-target effects of bone marrow transplantation chemotherapy, which may be important for understanding post-chemotherapy cognitive issues. Understanding the mechanism of peripheral macrophage engraftment is imperative to develop further cell-based gene therapy strategies for central nervous system diseases.“Microglial cells play an essential role in brain function and in the pathophysiology of many severe neurological diseases, both genetic and complex, such as multiple sclerosis and Alzheimer’s disease. Understanding the fate of these cells after the transplant process is essential both to clarify the consequences of chemotherapy and to develop new therapeutic strategies for serious neurodegenerative diseases,” said Nathalie Cartier, research director at Inserm and member of the NeuroGenCell team at the Brain Institute (ICM), and last co-author of the study.
“This study sheds light for the first time on a mechanism explaining how stem cell-derived macrophages enter the brain after bone marrow cell transplantation. This better understanding is essential to develop new strategies for gene and cell therapy applied to diseases of the central nervous system,” said Pierre-Marie Lledo, research director at the CNRS and head of the Perception and Memory Unit at the Institut Pasteur, and last co-author of the study.
“We show bone marrow transplantation chemotherapy to cause microglia, the brain’s resident immune cells, to lose their regenerative capacity. The microglia were unable to sustain their population which allowed bone marrow derived cells to replace them. This demonstrates how bone marrow transplantation is an effective therapy for certain neurological diseases and provides a strategy for cellular gene therapy in the central nervous system,” said Kurt Sailor, research fellow at the Institut Pasteur Unit Perception and Memory in Paris, and first author of the study.