Could the coronavirus epidemic that has hit China spread to Europe? A pertinent question given the new cases being announced by the Chinese authorities, of which eight have already been exported to other countries. An Inserm team led by researcher Vittoria Colizza at Pierre Louis Institute of Epidemiology and Public Health (Inserm/Sorbonne Université) has modeled the potential spread of 2019-nCoV in order to orient prevention and surveillance policies. A model which comes with one caveat: derived from research, its purpose is not to make predictions but rather be used as a theoretical tool to aid public decision-making.
As the situation is unfolding quickly, the following figures may also change, depending on the number of confirmed cases in and outside China. Vittoria Colizza’s model, based on the most recent figures up to 30 january, is now published on Eurosurveillance.
To follow the evolution of the number of cases, you can visit GISAID.
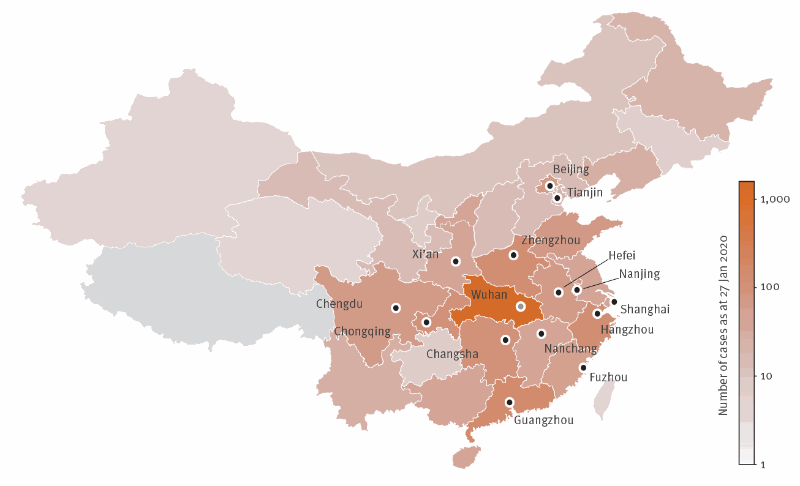
Just two weeks after announcing the discovery of a new virus from the coronavirus family responsible for severe pneumonia, there were 571 confirmed cases in China. In an effort to contain this epidemic whose death toll currently stands at 18, the Chinese authorities have taken drastic measures – particularly restrictions on travel from the province of Hubei, where the city of Wuhan is located.
Many questions continue to remain unanswered as to the origin of this new virus, 2019-nCoV, and about the capacity of the epidemic to spread to other regions of the world – particularly Europe. In the space of two weeks, eight cases have already been exported from China to Japan, South Korea, USA, Thailand and Taiwan.
From the start of the epidemic, Inserm researchers under the aegis of the research group REACTing have been working to model its potential dissemination.
Led by Inserm researcher Vittoria Colizza at the Pierre Louis Institute of Epidemiology and Public Health (Inserm/Sorbonne Université), a team is now able to propose a model to anticipate a potential arrival of the epidemic in Europe in order to guide surveillance and prevention measures. However, it is important to note that this model in no way constitutes a prediction of the future number of cases in France and Europe, but rather a theoretical tool to aid public decision-making.
Air traffic flows from China
When developing their model, the researchers focused on those Chinese provinces reporting more than ten cases. Their estimations of the risks of these cases being exported are based on January 2019 data on air traffic from these regions to Europe produced by the OAG – a world leader in flight data collection.
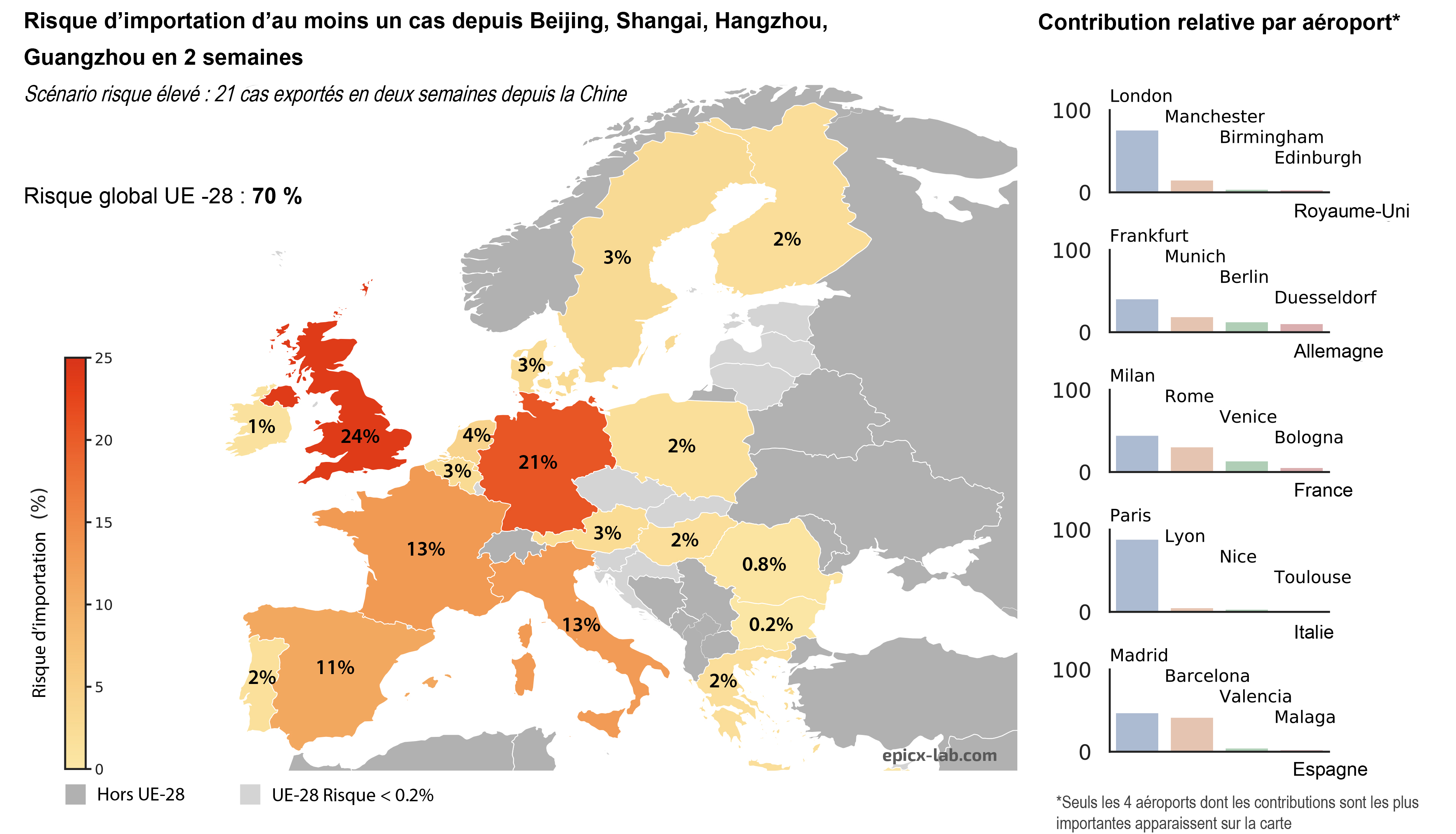
What is the risk of at least one case being imported to Europe in the next two weeks? The team addressed this question by preparing two scenarios: one of a low risk of dissemination and the other of a high risk.
The low-risk scenario is based on the situation (seven cases exported from China) prior to the flight ban by the Chinese government. It estimates the risk of at least one case being imported to Europe if seven cases are exported from the Chinese provinces affected in the next two weeks.
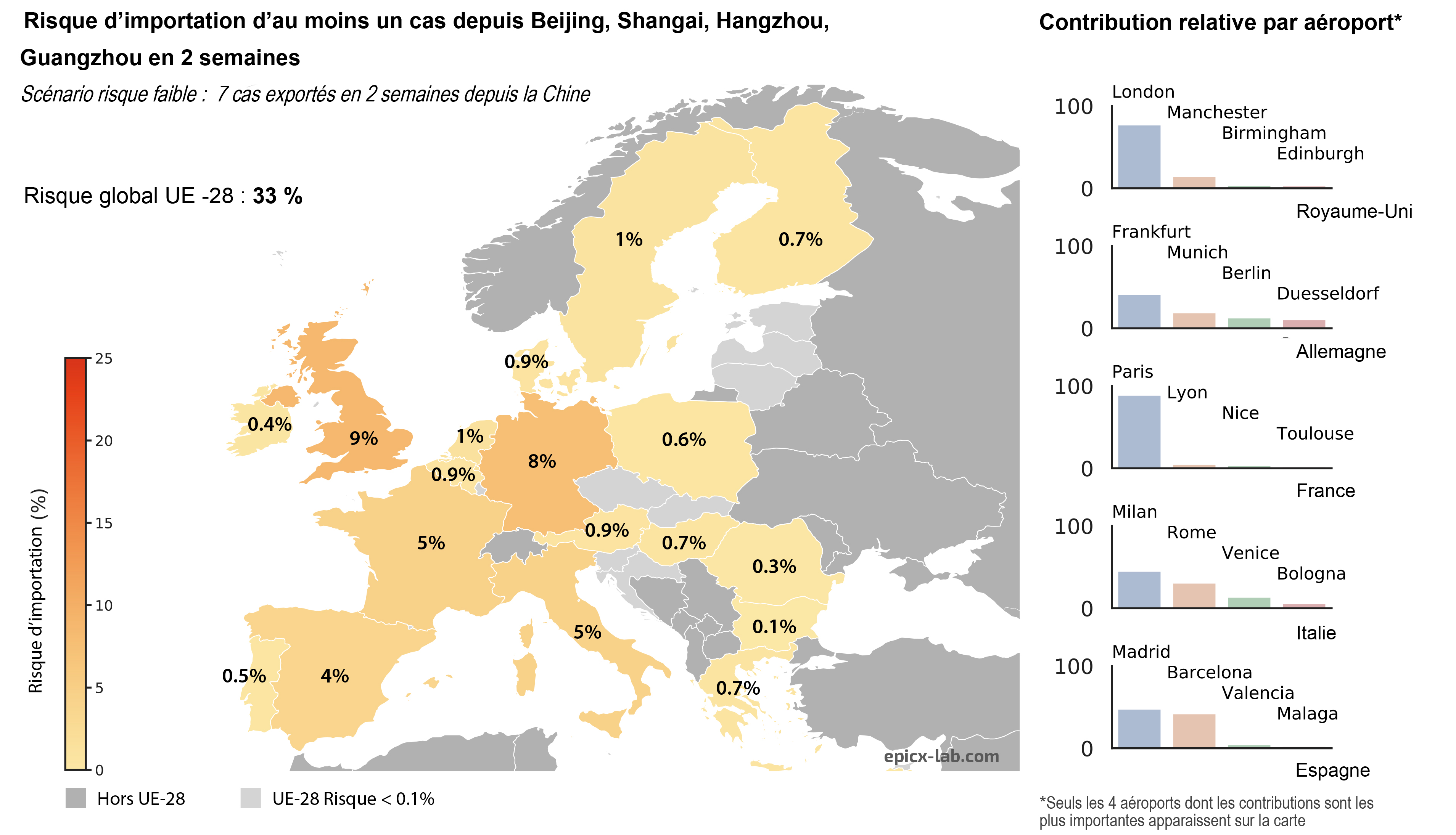
The high-risk scenario proposes an estimation of the same risk if three times more cases are exported from China. “It is an arbitrary choice but one which reflects the fact that the number of Chinese cases is on the increase, making it possible to anticipate the case of a greater number of infected people being exported”, emphasizes Colizza.