Researcher Contact
Mustapha Si-Tahar
Directeur de recherche Inserm
U1100 Centre d’étude des pathologies respiratoires (Inserm/Université de Tours)
E-mail : rf.mresni@rahat-is.ahpatsum
Téléphone sur demande
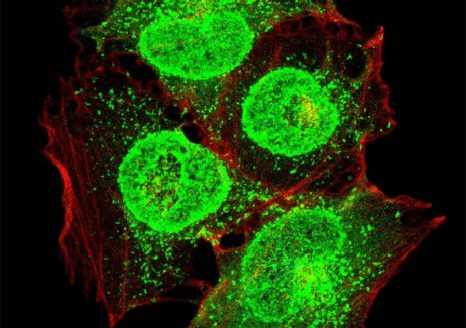
Human respiratory epithelial cells (in red) infected with an influenza virus (in green). © A. Cezard, D. Diakite, A. Guillon, M. Si-Tahar, PST ASB-Microscopy Department.
Seasonal influenza is a major public health issue because it continues to remain associated with considerable mortality, particularly among people who are elderly, immunocompromised, or both. It also has a significant socioeconomic cost. With vaccination and current treatments still being of limited efficacy, research teams are trying to develop new therapeutic approaches. At the Research Center for Respiratory Diseases in Tours, scientists from Inserm, Université de Tours and Tours Regional University Hospital have shown that in a context of influenza infection, a metabolite[1] called succinate, which is naturally present in the body, has an antiviral and anti-inflammatory action. These findings open up new therapeutic prospects based on the use of succinate derivatives. The study has been published in EMBO Journal.
Often considered a mild disease, influenza continues to cause the deaths of 10,000 to 15,000 people each year in France. The socioeconomic cost of the disease is also significant because it is associated with high levels of absenteeism and a major burden on hospitals.
Seasonal influenza vaccination is a central pillar of the preventive strategies deployed to reduce the number of cases and fight the disease. However, its efficacy can vary from year to year, depending on the influenza viruses in circulation and the suitability of the vaccine to them. Drugs that directly target the influenza virus are available for severe cases, but the window of time for effective action with these treatments is very short. What is more, influenza viruses have become resistant to their action.
In this context, the development of innovative therapies is a priority. While current treatments work by targeting certain components of the virus, Inserm Research Director Mustapha Si-Tahar and his colleagues at the Research Center for Respiratory Diseases are trying to better understand the host’s cellular and molecular responses to viral infection, with the long-term aim of developing novel therapeutic strategies aimed at strengthening these responses.
While metabolism1 has long been considered to be a purely energetic mechanism essential for cell function, recent research has shown that some metabolites can also regulate the immune response.
Based on these data, Si-Tahar’s team wondered whether an influenza infection could cause the reprogramming of the metabolism of the target cells of the virus and whether specific metabolites play an especially active role in the immune response.
They then exposed cells from the pulmonary epithelium to succinate, thereby demonstrating that the molecule has antiviral activity by blocking multiplication of the virus. Succinate also helps to reduce the strong inflammatory response that is triggered in the lungs following infection with influenza.
The researchers also found that mice exposed to the virus receiving intranasal succinate are better protected against infection and have a higher survival rate than those that do not receive it.
In order to better understand these different phenomena, the scientists sought to decipher the molecular mechanisms behind the antiviral action of succinate.
This involved analyzing the impact of succinate on the various stages of the viral replication cycle and demonstrating that, while there is no influence on the early stages of the cycle (entry, transcription, and translation), there is an influence on a later stage. The findings show that this metabolite prevents a major structural protein of the virus, the “nucleoprotein”, from exiting the nucleus of infected cells, thereby preventing the assembly of the final viral particle and interrupting the multiplication cycle of the virus.
Additional studies are needed in order to test the therapeutic potential of succinate and identify other metabolites of interest.
[1] Metabolism refers to all of the chemical reactions that take place inside the body’s cells. A metabolite is an organic substance derived from metabolism.
[2] In keeping with this research, Si-Tahar has since early 2021 coordinated a French National Research Agency (ANR) program entitled “Development of succinate-based formulations and analogues against SARS-CoV-2-induced respiratory infections and influenza viruses.” His team is also the beneficiary of an “ERS-RESPIRE4 Marie Skłodowska-Curie fellowship” to develop a project entitled “Succinate-Producing Probiotics as an Innovative Therapy for Viral Respiratory Infections: a proof-of-concept study”.
Mustapha Si-Tahar
Directeur de recherche Inserm
U1100 Centre d’étude des pathologies respiratoires (Inserm/Université de Tours)
E-mail : rf.mresni@rahat-is.ahpatsum
Téléphone sur demande
Host succinate inhibits influenza virus infection through succinylation and nuclear retention of the viral nucleoprotein
EMBO Journal, mai 2022
DOI : https://doi.org/10.15252/embj.2021108306
Antoine Guillon1,2,3, Deborah Bréa-Diakite1,2, Adeline Cezard1,2, Alan Wacquiez1,2, Thomas Baranek1,2, Jérôme Bourgeais2,4,5, Frédéric Picou2,4,5, Virginie Vasseur1,2, Léa Meyer6, Christophe Chevalier6, Adrien Auvet1,2,3, José M. Carballido7, Lydie Nadal Desbarats8, Florent Dingli9, Andrei Turtoi10,11,12, Audrey Le Gouellec13, Florence Fauvelle14,15, Amélie Donchet16, Thibaut Crépin16, Pieter S. Hiemstra17, Christophe Paget1,2, Damarys Loew9, Olivier Herault2,4,5, Nadia Naffakh18, Ronan Le Goffic6 and Mustapha Si-Tahar 1,2
1 Inserm, Centre d’Etude des Pathologies Respiratoires (CEPR), UMR 1100, Tours, France
2 Université de Tours, Tours, France
3 CHRU de Tours, Service de Médecine Intensive Réanimation, Tours, France
4 CNRS ERL 7001 LNOx Leukemic niche and redox metabolism, Tours, France
5 CHRU de Tours, Service d’Hématologie Biologique, Tours, France
6 Virologie et Immunologie Moléculaires, INRAe, Université Paris-Saclay, Jouy-en-Josas, France
7 Novartis Institutes for BioMedical Research, Basel, Switzerland
8 UMR 1253, iBrain, Université de Tours, Inserm, Tours, France.
9 Institut Curie, PSL Research University, Centre de Recherche, Laboratoire de Spectrométrie de Masse Protéomique, Paris, France
10 Tumor Microenvironment Laboratory, Institut de Recherche en Cancérologie de Montpellier, Inserm U1194, Montpellier, France
11 Institut du Cancer de Montpellier, Montpellier, France
12 Université de Montpellier, 34000 Montpellier, France