Press Contact
AstraZeneca France
Anne Leroux
Responsable communication scientifique, CVRM, Respiratoire & Immunologie
07 62 35 86 25
Inserm
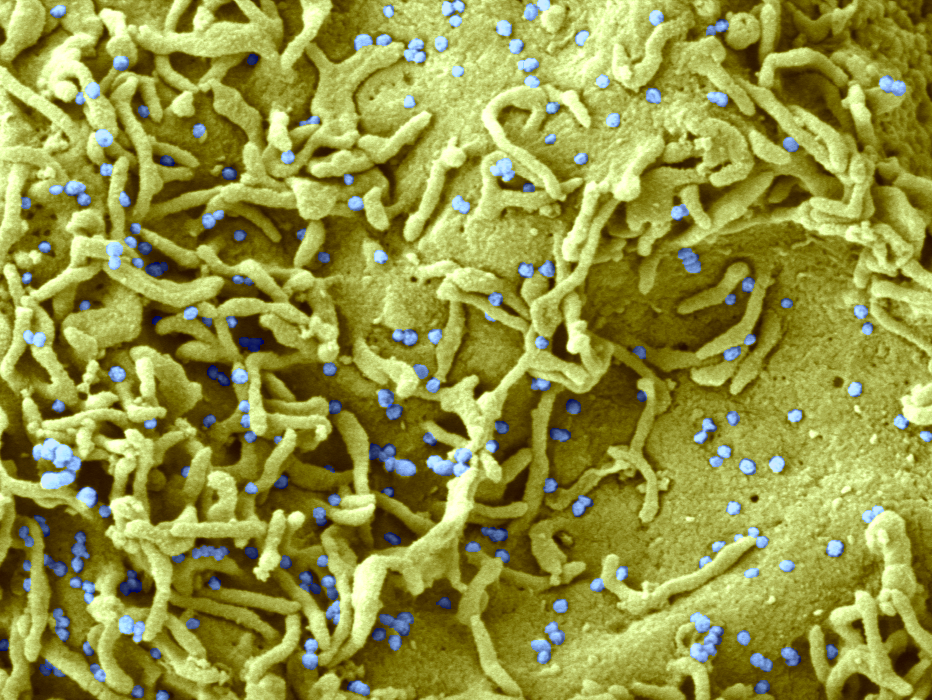
AZD7442 is a combination of two long-acting monoclonal antibodies (LAAB) derived from the plasma of two convalescent patients who have recovered from Sars-CoV-2 infection. © Electron microscopy of a cell infected with SARS-CoV-2 (Philippe Roingeard, Anne Bull-Maurer, Sonia Georgeault, unité Inserm U1259 MAVIVH & Université de Tours, France)
A long-acting antibody (LAAB) combination developed by AstraZeneca is to be evaluated in DisCoVeRy, the Inserm-coordinated European trial aimed at finding a treatment for COVID-19. It is planned to enroll 1240 patients across Europe in this Phase III clinical trial.
With its wealth of experience in the domain of targeted therapies (oncology, respiratory diseases), AstraZeneca has developed a combination of two monoclonal antibodies targeting the spike protein of SARS-CoV-2: AZD7442. This combination is now being clinically tested in various settings, both as a preventive and curative treatment for COVID-19.
International scientific commitments against Covid-19
DisCoVeRy, the clinical trial coordinated by Inserm, was set up in the very first months of the COVID-19 pandemic as a “daughter” trial of Solidarity – the international WHO trial with which it shares its data.
Jointly coordinated by Prof. Florence Ader, infectious disease specialist at Croix-Rousse Hospital of the Hospices Civils de Lyon (France) and Prof. Maya Hites, infectious disease specialist at Erasmus University Hospital in Brussels (Belgium), DisCoVeRy evaluates the clinical and virological efficacy and safety of a candidate treatment versus standard of care in adult patients hospitalized for Covid-19. The primary endpoint is patient clinical status at day 15[1]. An initial series of four treatments was tested. These were successively deemed ineffective on the basis of interim analyses, leading to the suspension of enrolments. The results of these analyses are currently being published in international peer-reviewed journals and those concerning the first three treatments are also available on the medRXive website.
Funded by Europe (European Union Horizon 2020 program for research and innovation), Discovery is now Work Package 1 of the European Research and Preparedness Network for Pandemics and Emerging Infectious Diseases (EU-RESPONSE) funded by the EU Horizon 2020 research and innovation program, bringing together 21 partners (clinics, hospitals, universities…) from 13 European Union countries, plus Norway, Switzerland, and Turkey). Created in response to the COVID-19 epidemic emergency, EU-RESPONSE, coordinated by Prof. Yazdan Yazdanpanah, also makes it possible to expand DisCoVeRy to involve other European countries and build a sustainable clinical trial platform to fight COVID-19 followed by any other emerging epidemics.
It was within this academic framework that the partnership between Inserm, through its private subsidiary Inserm Transfert, and AstraZeneca to evaluate a promising new treatment for SARS-CoV-2 was born.
AZD7442
Developed by AstraZeneca for Covid-19 patients, AZD7442 is a combination of two monoclonal long-acting antibodies (LAABs) derived from the plasma of two convalescent patients following SARS-CoV-2 infection. These antibodies directed against the S protein of the virus were selected for their ability to neutralize it in a synergistic manner. In addition, these antibodies that recognize two distinct parts of the S protein are less likely to be affected by mutations concerning that protein.
This combination of antibodies is administered as a single dose by parenteral route with the objective of limiting severity of infection in patients at risk of developing a severe form.
AstraZeneca is already evaluating AZD7442 in other clinical trials: as a preventive treatment in PROVENT and STORM CHASER and as a curative treatment in TACKLE, ACTIV-2, and ACTIV-3.
The randomized, multicenter, double-blind, placebo-controlled DisCoVeRy trial will therefore make it possible to test the efficacy of AZD7442 in hospitalized patients with Covid-19.
The first inclusion is taking place in France at the beginning of this week, followed by those in the partner countries.
The efficacy of the treatment will be assessed on the basis of the following criteria:
The 1240 patients enrolled in the study in Europe will be followed up over a 15-month period until November 2022. An initial analysis of the results is expected to take place at the end of 2021.
“We hope that this treatment will help reduce disease severity and will improve the clinical condition of hospitalized patients” conclude Prof. Florence Ader and Prof. Maya Hites, co-lead investigators of the study.
“This collaboration is a very good example of a public/private partnership in which the relevance of a therapeutic developed by an industrial player can be validated by academic teams with internationally recognized expertise in the field. AstraZeneca is very proud to participate in this trial, that will bring a curative treatment of Covid-19 to hospitalized patients, if positive,” concludes Professor Gabriel Thabut, Medical Director Respiratory and Immunology at AstraZeneca.
[1]Measured on the WHO 7-point ordinal scale
AstraZeneca France
Anne Leroux
Responsable communication scientifique, CVRM, Respiratoire & Immunologie
07 62 35 86 25
Inserm