The European Commission is legally required to provide criteria identifying Endocrine Disrupting Chemicals (EDCs), a process that has been blocked for almost three years, allegedly because of a lack of scientific consensus and because an impact assessment study was deemed necessary. Now, a group of 7 independent researchers from universities and research institutions from Europe and the United States* show how little controversy there is around the definition of EDCs, and that the simple logic used for the identification and regulation of carcinogens can be used for EDCs. The study is published this Monday as a commentary in the scientific journal Environmental Health Perspectives.
First, the authors demonstrate that there is wide acceptance of the World Health Organization (WHO) definition of an EDC as an exogenous substance or mixture that alters function(s) of the endocrine system [i.e. the hormonal system] and consequently causes adverse health effects in an intact organism or its progeny, populations or subgroups of the population.
Second, the authors describe the approach used for the identification of other health hazards of equivalent concern, such as carcinogens or reproductive toxicants. This identification relies on a simple categorization with 3 levels not referring to the toxicological concept of potency[1]. A similar approach not relying on potency should be used for endocrine disruptors; the 3 categories proposed by the European Commission as one of the options considered correspond to “endocrine disruptors”, “suspected endocrine disruptors” and “endocrine active substances” (substances altering the endocrine system with no evidence of the induction of an adverse health effect); these categories are judged sufficient by the researchers. Inclusion of potency or dose-response considerations would modify the spirit of the pesticides and biocides laws, which call for a hazard-based (and not a risk-based) management of pesticides if exposure is not negligible.
Finally, they state that it is not defensible to conduct an impact assessment study to determine scientific criteria. This would be a dangerous precedent, since impact assessment studies are not meant to define hazards, but to quantify the health, social and economic impacts of regulation. This is in line with the decision of the European Court of Justice (2015), which stated that “the definition of scientific criteria to identify properties disrupting the endocrine system can only be done in an objective manner based on scientific data relative to the endocrine system, independently from any other consideration, and in particular from any economic consideration”.
Authors recognize that scientific uncertainty remains with regard to the finer detail of mechanisms, the exact extent of health and environmental effects of EDCs and their impact at the population level. There are also questions around the number of substances likely to be identified as EDCs. However, filling these knowledge gaps is not required to provide scientific criteria defining EDCs.
Several years have been spent trying to issue scientific criteria defining a hazard that was actually defined some time ago (in 2002) by a state-of-the-science report from WHO. For this reason, the scientists consider that the claim of a lack of consensus among scientists was forged to justify delays in the publication of the scientific criteria. Deferring the publication of the scientific criteria can be seen as a way to postpone full application of the 2009 pesticide and 2012 biocide laws. Authors insist that impact assessment studies should not be used as an argument for postponing the publication of a scientific definition. They express concern that scientific definitions might be distorted in order to modify the spirit of a law, thereby muddling science and policy, and postpone the application of existing laws. This postponement is all the more worrying since these scientific criteria are but one of the first steps towards identifying EDCs and providing more efficient protection of public health in the European Union.
Background information
Endocrine Disrupting Chemicals (EDCs) can be very diverse in terms of chemical nature, origin or occurrence. Suspected EDCs include metals (e.g., mercury), organochlorine pesticides such as DDT or triclosan (used e.g. in tooth paste or soaps), other pesticides, dietary contaminants such as bisphenol A, phenols such as parabens (used as preservatives in cosmetics) or phthalates, which can be found in perfumes, cosmetics, medical devices, polyvinyl chloride (PVC) plastics, rainwear… Effects on health outcomes such as congenital malformations, neurodevelopment, behaviour, breast cancer, have been reported for some of these substances in animal models or human studies. The resulting health-related costs in the European Union is estimated to be in the €100-200 billion range (Trasande L et al., JCEM, 2015).
Europe is the only large economy in the world with an ambitious legislation on EDCs. In addition to other health hazards such as carcinogens, mutagens or reproductive toxicants, the European Parliament has identified EDCs as a new type of hazard for health and the environment.
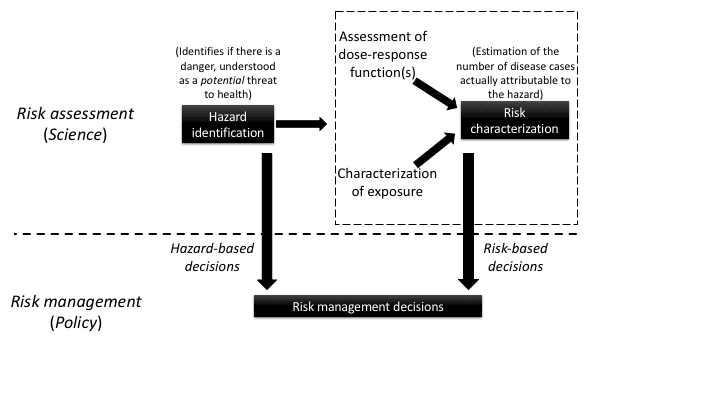
A strategy on endocrine disruptors exists in the European Union since 1999, and in 2009 and 2012, the European Parliament passed two laws on pesticides and biocides (the Plant Protection Products [pesticides] Regulation in 2009 and the Biocide Products Regulation in 2012). These laws stipulated that, for compounds for which population exposure is not negligible, pesticides and biocides containing endocrine disruptors should be regulated on a hazard-based logic (as opposed to a risk-based logic). This implies that there is no need to characterize in detail the dose-response function, identify all possible health effects induced, or possible thresholds in the effects of the compound on health. This approach allows faster management of health risks and more limited resort to test animals, and avoids the debate on the plausibility of the existence of thresholds in the action of these compounds. It is also the logic planned for the management of pesticides and biocides containing carcinogens, mutagens and reproductive toxicants.
The application of the part of these laws regarding endocrine disruptors required the European Commission to publish criteria for identifying endocrine disruptors not later than 2013. This has not been done. Instead, a Roadmap listing 4 options to identify criteria was published by the European Commission in 2014 and the start of an impact assessment study aiming at supporting the choice between the 4 options announced. In December 2015, the European Court of Justice ruled that an impact assessment study was irrelevant, and urged the Commission to publish criteria without delay. The assessment done by the researchers point to the “Option 3” of the European Commission roadmap as being scientifically relevant and operational.
Since 2013, the pesticide and biocide laws (for their part relative to endocrine disruptors) could not be applied because of the lack of publication of criteria for identifying endocrine disruptors. The European Commission indicated in February 2016 that two documents, including one providing criteria for the identification of EDCs, would be published by summer 2016.
Note that, after submission of this publication, a distinct consensus workshop was held by scientists on April 11-12, 2016 in Berlin on a similar topic. The workshop was focused on more fundamental scientific notions, and concluded on the relevance of the WHO definition of EDCs, and the lack of relevance of the concept of potency to identify EDCs. More information can be found on the BfR website.
APPENDIX
Distinction between hazard-based and risk-based regulation of chemicals (copyright RemySlama-Inserm)
* Authors are affiliated with Inserm- Université Grenoble Alpes and CNRS-Museum national d’histoire naturelle (France); CHU Liège (Belgium) ;University of Nottingham and Brunel University London (UK), University of Torino (Italy); University of Massachusetts (USA)
[1] According to the International Union of Pharmacology, potency is « an expression of the activity of a drug [or toxic substance], in terms of the concentration or amount needed to produce a defined effect; an imprecise term that should always be further defined » (Neubig, Pharmacological Reviews, 2003). Researchers point to the vagueness of this definition and recommend to use the more informative and related concept of dose-response function.
These contents could be interesting :
- Inserm, CNRS and Univ. Grenoble Alpes, IAB joint research center, Team of Environmental Epidemiology, Grenoble, France.
- Pediatric Endocrinology, CHU Liège and Neuroendocrinology Unit, GIGA Neurosciences, Univ. Liège, Belgium.
- UMR CNRS/MNHN 7221, Dept. RDDM, Muséum National d'Histoire Naturelle, 75005 Paris, France.
- School of Biosciences & School of Veterinary Medicine and Science, University of Nottingham, UK.
- Dept. Neuroscience, University of Torino, and Neuroscience Institute Cavalieri Ottolenghi (NICO), Orbassano, Italy.
- Brunel University London, Institute of Environment, Health and Societies, Uxbridge,UK.
- University of Massachusetts, Biology Department, Amherst (MA), USA.