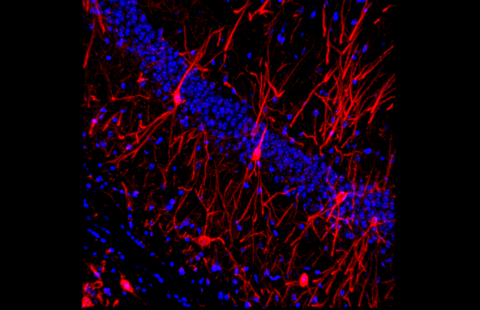
Motoneurones (stained by immunohistochemistry for ChAT) of the lumbar section of the spinal cord in ALS mouse models. Scale 100 mm © Simon J Guillot, Daniel Beckett ,Matei Bolborea
Motoneurones (stained by immunohistochemistry for ChAT) of the lumbar section of the spinal cord in ALS mouse models. Scale 100 mm © Simon J Guillot, Daniel Beckett ,Matei Bolborea
Amyotrophic Lateral Sclerosis (ALS), Charcot’s disease, or Lou Gehrig’s disease is a severe neurodegenerative disease that leads to progressive paralysis of muscles involved in voluntary movement. To date, no curative treatment exists. The disease is typically fatal within three to five years of onset. Researchers from Inserm and the University of Strasbourg, at the Center for Biomedical Research, have made progress in understanding the mechanisms underlying ALS. In a new study, they reveal that characteristic symptoms of the disease are preceded by sleep disturbances. Their findings suggest that sleep disorders appear before the onset of motor impairment and respiratory issues. The study highlights a previously unknown role of certain hypothalamic neurons in the emergence of these ALS-related sleep disturbances. Published in Science Translational Medicine, this research identifies new potential therapeutic targets in the brain and explores a novel class of molecules to counteract the effects of sleep deprivation.
ALS results from the progressive death of nerve cells known as motor neurons. This degeneration leads to rapid and progressive muscle atrophy, resulting in a loss of autonomy due to motor dysfunction and deficits. In most cases, respiratory muscle failure is the primary cause of death.
In recent years, research efforts have significantly advanced the understanding of the genetics and biology of ALS. However, pinpointing the precise mechanisms that initiate and sustain neuronal degeneration remains a challenge. Unraveling these mechanisms is a key research objective for the team led by Luc Dupuis and Matei Bolborea, both Inserm researchers at the Strasbourg Center for Biomedical Research (Inserm/University of Strasbourg).
As ALS progresses, it affects respiratory function, indirectly leading to sleep disturbances. However, it was unclear whether these disturbances could precede motor symptoms, as observed in several other neurodegenerative diseases like Parkinson’s and Alzheimer’s. When do these disturbances emerge? Are they a consequence of motor dysfunction? These questions were investigated by the team of Luc Dupuis and Matei Bolborea in close collaboration with the German Center for Neurodegenerative Diseases (DZNE) in Ulm.
In their latest research, the scientists collected and analyzed dozens of sleep recordings from individuals with ALS at different stages of the disease, comparing them to control groups.
One group consisted of ALS patients who had not yet developed respiratory symptoms. Another group included presymptomatic individuals carrying specific genetic mutations associated with the disease, meaning they were at increased risk of developing ALS.
The results indicated that both groups experienced similar sleep disturbances: increased wakefulness and a reduced amount of deep sleep compared to the control groups.
These findings suggest that sleep disturbances are present and observable long before the onset of motor symptoms—potentially several years in advance.
Restoring Sleep in ALS Patients
Following this discovery, researchers sought to determine the origin of these sleep disturbances in the brain. They focused on specific neurons in the hypothalamus known as orexin[1] neurons, which play a crucial role in wakefulness. Using ALS mouse models, the team observed the same types of sleep disturbances. They also discovered that neuronal circuits involving orexin neurons were disrupted due to the loss of supporting neurons as the disease progressed.
This led them to test an orexin-inhibiting compound found in an already approved sleep medication prescribed for insomnia[2]. Administering this drug to ALS-affected mice successfully restored sleep with a single oral dose. Furthermore, restoring the activity of neurons supporting orexin neurons led to motor neuron preservation after 15 days of treatment.
A clinical trial is currently underway to test this compound in ALS patients. The goal is to determine whether restoring sleep can influence the progression of the disease.
‘Our main objective was to unveil what happens in patients before they show any motor symptoms,’ explains Matei Bolborea, co-senior author of the study.‘ These discoveries about the timeline of symptoms allow us to reconsider the brain’s role—particularly the hypothalamus—in the onset of ALS and to explore new therapeutic targets.‘
Luc Dupuis, co-senior author of the study, adds: ‘Our team’s findings are significant on two levels. First, they shed light on a new timeline of ALS symptoms, prompting us to reevaluate the origins of the disease and the brain’s role in its development. Second, they offer a glimmer of hope for patients, as addressing the early manifestations of the disease might slow its otherwise extremely rapid progression.’
[1] Studies have shown, for example, that people with narcolepsy have fewer orexin neurons than others.
[2] Suvorexant® belongs to a class of drugs known as hypnotics, and is approved for sale in the USA.
These contents could be interesting :