Researcher Contact
Laurent Reber
Unité Inserm 1291 Infinity, 5051 CNRS, Université Toulouse 3 – Institut Toulousain des Maladies Infectieuses et Inflammatoires
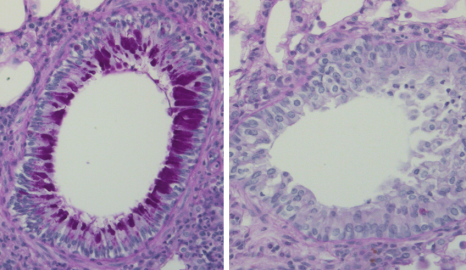
Visualisation au microscope de coupes de poumon de souris dans un modèle d’asthme avec une coloration à l’acide périodique et Schiff montrant une forte production de mucus (violet foncé) et un infiltrat de globules blancs autour des bronches dans le groupe contrôle (gauche) mais pas dans le groupe vacciné (droite). © Dr Eva Conde
Inserm teams led by Laurent Reber (Infinity, Toulouse) and Pierre Bruhns (Humoral Immunity, Institut Pasteur, Paris) and French company NEOVACS have developed a vaccine that could induce long-term protection against allergic asthma, reducing the severity of its symptoms and thus significantly improving patient quality of life. Their research in animals has been published in the journal Nature Communications.
Asthma is a chronic disease affecting around 4 million people in France and 340 million worldwide. Allergic asthma is characterized by inflammation of the bronchial tubes and respiratory discomfort caused by the inhalation of allergens, most often dust mites. This exposure to dust mites and other allergens leads to the production of antibodies called immunoglobulin E (IgE) and type 2 cytokines (such as interleukin-4 (IL-4) and IL-13) in the airways. This leads to a cascade of reactions resulting in hyperresponsiveness of the respiratory tract, overproduction of mucus, and eosinophilia (when there are too many eosinophils, a type of white blood cell, in the airways).
Inhaled corticosteroids are the gold standard for controlling asthma. However, in the case of severe asthma, this treatment is not enough. The use of therapeutic monoclonal antibodies that target IgE or the IL-4 and IL-13 pathways is then required. However, these are costly and require the long-term or even lifelong administration of injections.
To overcome this problem, researchers from Inserm, Institut Pasteur, and the company NEOVACS, have developed a conjugate vaccine, called a kinoid, by coupling the recombinant cytokines IL-4 and IL-13 with a carrier protein called CRM197 (a non-pathogenic mutated form of the diphtheria toxin, used in many conjugate vaccines).
The preclinical results (in animal models) show that this vaccine induces the sustained production of antibodies specifically directed against IL-4 and IL-13. Indeed, six weeks after the first injection of the conjugate vaccine, 90% of the mice presented high levels of antibodies. Over one year after primary immunization, 60% of them still had antibodies capable of neutralizing IL-4 and IL-13 activity.
The researchers also showed an effect on asthma symptoms: the vaccine was able to strongly decrease levels of IgE, eosinophilia, mucus production and airway hyperresponsiveness in a model of dust mite allergic asthma. This study therefore suggests both the prophylactic and therapeutic efficacy of the vaccine in this model of asthma and no adverse effects were observed in the animals.
The research team’s findings will now need to be tested in a clinical trial setting.
Laurent Reber
Unité Inserm 1291 Infinity, 5051 CNRS, Université Toulouse 3 – Institut Toulousain des Maladies Infectieuses et Inflammatoires
Nature Communications