Neisseria meningitidis, also called meningococcus, is a bacterium responsible for meningitis and septicemia[1]. Its most serious form, purpura fulminans, is often fatal. This bacterium, which is naturally present in humans in the nasopharynx, is pathogenic if it reaches the blood stream. Teams led by Dr. Sandrine Bourdoulous, CNRS senior researcher at the Institut Cochin (CNRS/Inserm/Université Paris Descartes), and Professor Xavier Nassif, Institut Necker Enfants Malades (CNRS/INSERM/Université Paris Descartes/Assistance Publique – Hôpitaux de Paris), have deciphered the molecular events through which meningococci target blood vessels and colonize them. This work opens a path to new therapeutic perspectives for treating vascular problems caused by this type of invasive infection. The study was published on June 1, 2014 in Nature Medicine.
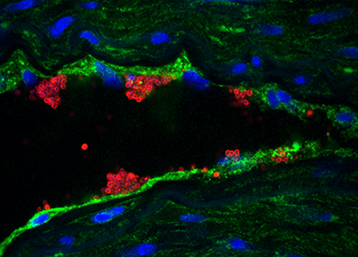
Colonization of brain vessels by N. meningitidis Immunofluorescence analysis of a human brain section infected by N. meningitidis. The bacteria (red) have colonized the brain’s endothelial cells that express CD147 (green). (Cell nuclei are blue) © Nature Medicine
When the bacterium Neisseria meningitidis multiplies in the blood, it interacts with the endothelial cells that line the inside of blood vessels and adheres to their walls. In the skin and mucous membranes, meningococcal infection in the vessels creates hemorrhagic skin lesions (called purpura) due to bleeding in the tissues. Those can rapidly progress to a serious and often fatal form of the disease (purpura fulminans). In the brain, when meningococci adhere to the vessels they can pass through the blood-brain barrier[2], and cause meningitis when they invade the meninges[3].
Teams of researchers have deciphered how Neisseria meningitidis adheres to blood vessels, a step that underpins the bacterium’s pathogenicity. In blood vessels they have identified receptor[4] CD147, whose expression is essential for initial meningococcal adherence to endothelial cells. If this receptor is absent, N. meningitidis cannot implant in blood vessels and colonize them.
It is a well-known fact that the adherence process of meningococcal bacteria to human cells relies on pili, long filaments that are expressed by the bacterium and composed of different sub-units (pilins). However, the pilins specifically involved in N. meningitidis’ adherence to blood vessels had never been identified. The researchers have determined that two pilins, PilE and PilV, interact directly with the CD147 receptor. Without them, meningococci cannot adhere to endothelial cells.
Humans are the only species that can be infected by meningococci. To show in vivo that pilins PilE and PilV are essential for N. meningitidis to colonize the vascular network, the researchers used a mouse model, where the mice were immunodeficient and grafted with human skin, keeping the functional human vessels within the graft to reproduce in mice the infection stages as observed in human skin. These mice were then infected by meningococci naturally having pilins PilE and PilV, or meningococci in which the expression of these pilins had been artificially suppressed. The human blood vessels were only infected by meningococci displaying PilE and PilV, which confirms that these two pilins are essential to the bacterial colonization process.
The researchers also showed in an ex vivo[5] infection model that cerebral vessels and meninges, particularly rich in CD147 receptors, allow colonization by meningococci, unlike other parts of the brain.
The scientists now wish to develop a new type of vaccine (to complement those already available) that would block the interaction between N. meningitidis and the CD147 receptors, thereby stopping the bacterium from colonizing the vessels.
This study was made possible by support from the teams of Dr. Frank Lafont at the Centre d’Infection et d’Immunité in Lille (CNRS/INSERM/Institut Pasteur de Lille/Université Lille 1/Université Lille 2), Professor Fabrice Chrétien at the Unité Histopathologie Humaine et Modèles Animaux at the Institut Pasteur in Paris, and Dr. Eric Chevet in the Groupe de Recherches pour l’Etude du Foie (INSERM/Université de Bordeaux).
[1] Systemic infections
[2] The endothelial cells in the brain’s capillaries are a physiological barrier at the interface between the blood and the brain (the blood-brain barrier). These cells, which have unique properties, act as a selective filter through which the necessary energy sources are transmitted to the brain and the waste is removed. Therefore they protect the brain from the external environment, including pathogens.
[3] Envelopes that protect the central nervous system.
[4] A receptor is a protein in the cell membrane onto which a specific factor (a ligand) can bind, triggering a response in the cell.
[5] This expression refers to culture tissues and live cells made in the laboratory, outside the organism they came from.