To evaluate the safety and efficacy of potential COVID-19 vaccines, rigorous clinical trials are more necessary than ever. © Adobe Stock
The search is on for a COVID-19 vaccine, with many candidates having already reached the clinical trial stage. The participation of France in such trials is scientifically and strategically imperative in order to guarantee access to a safe and effective vaccine for its population. This is why Inserm, with the support of the REACTing network, Public Health France, the country’s university hospitals, and the French College of Teachers in General Practice, is launching COVIREIVAC. This platform for the clinical evaluation of COVID-19 vaccine candidates will make it possible to test them rigorously and obtain robust data on their safety and ability to induce an immune response (immunogenicity).
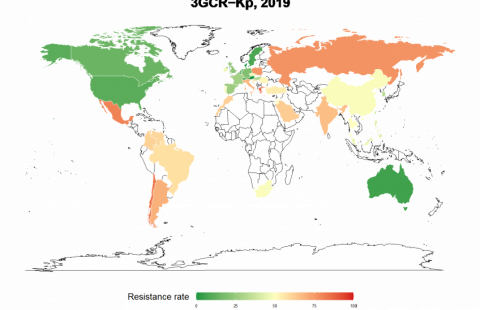
Unprecedented in scale, the COVID-19 pandemic has already caused more than 500,000 deaths worldwide. While the number of new cases is slowing in some regions, the virus continues to spread, particularly in the USA and Latin America. In order to stop this, the scientific community is pinning its hopes, first and foremost, on the development of a vaccine. Several months after the identification and genetic sequencing of SARS-CoV-2, the World Health Organization (WHO) has listed over 140 vaccine candidates, seventeen of which are already in clinical development.
In order to assess the safety and efficacy of these potential vaccines, rigorous clinical trials are more necessary than ever.
Founded by Inserm in 2007 and labeled a network of excellence in 2013 by its national infrastructure F-CRIN[1] , the Innovative Clinical Research Network in Vaccinology (I-REIVAC) enjoys extensive clinical vaccine research experience and industry visibility, making it an essential stakeholder in the organization of such trials.With 24 hospital clinical centers distributed throughout France, it also enables extensive participation of the population in the vaccine trials. On the strength of this legitimacy, this network will serve as the support structure for the COVIREIVAC project, which should enable high-quality clinical evaluation of the various COVID-19 vaccine candidates.
A “one-stop shop” for France
This project is based on the observation that France’s access to the most promising candidates will only be possible by setting up a “one-stop shop” serving industry and academia, in order to evaluate these products, guarantee the feasibility of the clinical trials to the industry stakeholders concerned, and negotiate production and market conditions. This approach involves both the establishment of a “scientific committee” tasked with carrying out scientific and strategic evaluation of the various COVID-19 vaccine candidates, and the development of a national platform for their clinical evaluation.
Chaired by Inserm Research Director and CARE committee member Marie-Paule Kieny, the scientific committee is particularly interested in the characteristics of the vaccine candidates that could provide indications regarding their efficacy, safety and production capacity in order to identify the most relevant products. It is working closely with the new vaccine platform, under the leadership of I-REIVAC coordinator Odile Launay, professor of infectious and tropical diseases at Université de Paris and coordinator of the Cochin Pasteur Clinical Investigation Center (CIC) at Cochin Hospital (AP-HP), to test vaccines in rigorous clinical trials that can include several hundred participants.
With the support of the REACTing network, Public Health France, the university hospitals and the French College of Teachers in General Practice, and in order to increase the capacity of the network to participate in COVID-19 vaccine trials, the aim of the platform will be to build a pool of potential participants and increase the number of centers that can accommodate them.
The monitoring of potential side effects, through close collaboration with networks of primary care doctors and the French medicines agency (ANSM), will also be proposed.
“Confronting the severity of the COVID-19 pandemic means ensuring the widest possible access to future vaccines for the French and European populations. Thanks to an existing vaccine network, the COVIREIVAC project can give France the attractiveness it needs to guarantee active participation in the major vaccine trials and early access to the best vaccine candidates,” says Gilles Bloch, Inserm Chairman and CEO.
In the longer term, the initiative will also provide essential information on the safety of these vaccines and their continued efficacy. Collaboration with other European countries, to extend the initiative and enable larger-scale clinical trials, is also something that is being envisaged.
[1] French Clinical Research Infrastructure Network
These contents could be interesting :