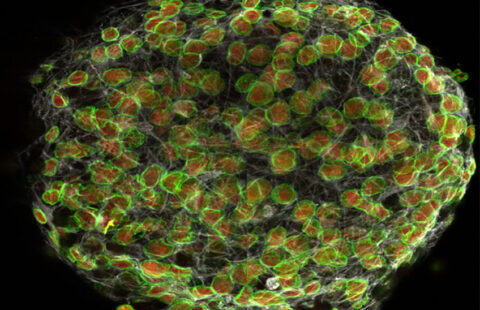
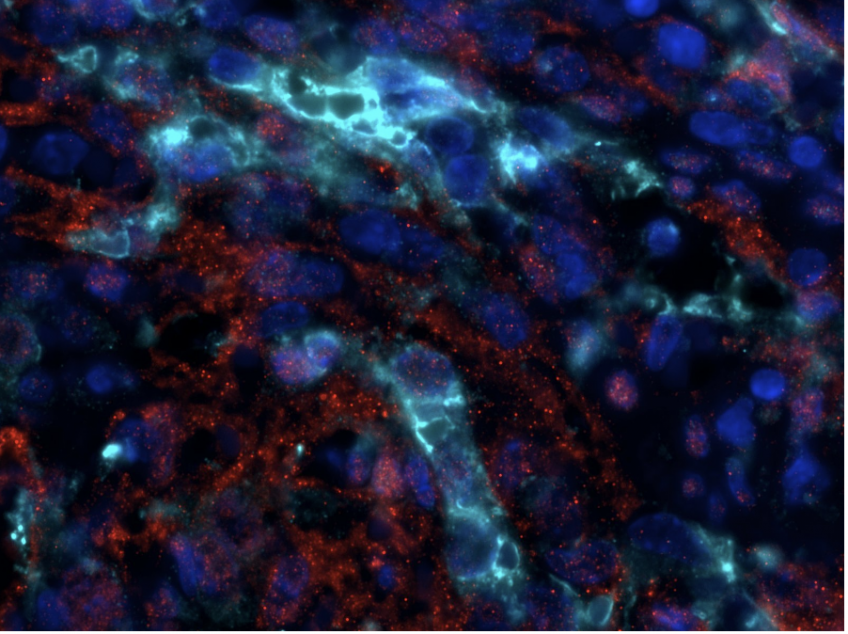
 Phospho-ERK (red), Green Fluorescent Protein (cyan) and DAPI coimmunofluorescence on spleen sections from mice carrying a KRAS G12C endothelial mutation © Guillaume Canaud
Phospho-ERK (red), Green Fluorescent Protein (cyan) and DAPI coimmunofluorescence on spleen sections from mice carrying a KRAS G12C endothelial mutation © Guillaume Canaud
The teams of the translational medicine and targeted therapies unit of the Necker-Enfants Malades AP-HP hospital, Inserm, Paris Cité University within the Necker–Enfants Malades Institute, coordinated by professors Guillaume Canaud (Université Paris Cité, AP-HP) and Laurent Guibaud (Hospices Civils de Lyon, Reference Center for Superficial Vascular Anomalies), conducted a study showing a promising effect of the anticancer drug sotorasib for arteriovenous malformations secondary to a mutation in the G12C type KRAS gene. The results were the subject of a publication published on July 17, 2024 in the New England Journal of Medicine.
An arteriovenous malformation (AVM) results from abnormal connections between arteries and veins. AVMs are frequently associated with symptoms such as pain, bleeding, heart failure, cosmetic deformity or organ compression. These malformations generally progress over time. AVMs are in most cases of genetic origin, either “germinal” and therefore familial, or sporadic due to a localized genetic mutation. In many of the latter cases, the gene responsible is the KRAS gene, a gene involved in cell growth, proliferation and survival. There are different types of KRAS mutation. At the moment, no drug treatment is approved for these pathologies.
Sotorasib, developed by Amgen, is an anticancer drug used to treat a type of lung cancer, advanced non-small cell lung cancer (NSCLC), with a mutation in the KRAS gene, the KRAS G12C mutation. The drug thus selectively targets the KRAS p.G12C protein.
The teams of Professors Guibaud and Canaud identified the presence of a KRAS G12C mutation in two adult patients with severe AVMs and without therapeutic resources. They then decided to create two mouse models developing vascular malformations secondary to a KRAS G12C mutation in order to better understand the pathophysiology of these malformations. Preclinical models have largely recapitulated the malformations observed in humans. Using these two models, they then tested and demonstrated the effectiveness of sotorasib in preventing the development of vascular malformations and significantly improving the survival of mice.
Based on these results, Professors Guibaud and Canaud obtained authorization for the use of sotorasib from the Amgen laboratory to administer it to these two patients as part of their therapeutic care.
In the weeks following the start of treatment, both patients noted a clear improvement in their symptoms (stopping of bleeding, healing of chronic skin ulcerations, disappearance of pain and recovery of deafness), a clinically visible reduction in malformation. This last result was then confirmed by MRI. After 2 years of follow-up, patients did not develop resistance to treatment.
This work demonstrates the interest in obtaining a molecular diagnosis for this type of rare disease and the possibility of repositioning highly targeted drugs developed for other indications, as the researchers had previously done for alpelisib in the syndrome of CLOVES and related syndromes.
These results will need to be confirmed by future studies on a larger number of patients. These drug repositionings open up new therapeutic fields, in particular for AVMs, for which these drugs could be combined with surgical treatment or in interventional radiology.
These contents could be interesting :
Sotorasib for Vascular Malformations Associated with KRAS G12C Mutation
New England Journal of Medicine
DOI: 10.1056/NEJMoa230916
Antoine Fraissenon, Charles Bayard, Gabriel Morin, Sandro Benichi, Clément Hoguin, Sanela Protic, Lola Zerbib, Sophia Ladraa, Marina Firpion, Thomas Blauwblomme, Olivier Naggara, Michael Duruisseaux, Marion Delous, Clothilde Boitel, Pierre-Paul Bringuier, Léa Payen, Christophe Legendre, Sophie Kaltenbach, Estelle Balducci, Patrick Villarese, Vahid Asnafi, Annouk Bisdorff, Laurent Guibaud, Guillaume Canaud