Researcher Contact
Julie Guignot
Institut Cochin
E-mail : whyvr.thvtabg@vafrez.se
Téléphone : 01 40 51 64 13
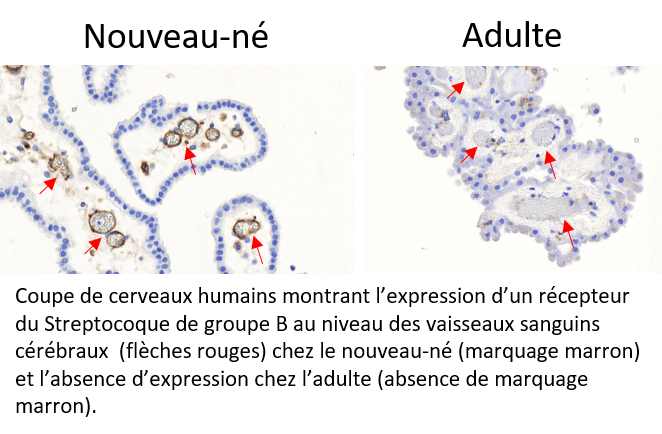
Every year throughout the world, Group B Streptococcal (GBS) meningitis affects thousands of newborns. Often fatal, the disease can also lead to severe after-effects in survivors. However, in adults, GBS is an uncommon cause of meningitis. Researchers from Inserm, Collège de France, CNRS, Institut Pasteur, Université de Paris and Paris hospitals AP-HP have now provided elements to explain the predisposition of newborns to GBS meningitis. They have identified and demonstrated that receptors for a bacterial protein enabling penetration of the blood-brain barrier[1] are overexpressed in newborns and absent in adults. The results of their research have been published in Journal of Clinical Investigation.
Group B Streptococcus (GBS) is present in the vaginal microbiota of 20-30% of women. To avoid infecting the baby during labor – which could lead to septicemia and, in the severest cases, meningitis – many developed countries, including France, perform vaginal screening a few weeks before birth. Women found to carry GBS are then given antibiotics during labor.
While this strategy has led to a strong reduction in the incidence of GBS infections during the first week of life, it has had no effect on those occurring between 1 week and 3 months of life.
What is more, many countries offer no such prenatal screening and large numbers of newborns die of GBS meningitis. It is therefore a major public health problem.
Predisposition of newborns
In order to better understand the disease and improve the care of mothers and children, Inserm researcher Julie Guignot and her team at Institut Cochin (Inserm/CNRS/Université de Paris)[2] sought to understand what predisposes newborns to this type of meningitis that affects children and adults only in exceptional cases.
In previous research, the scientists had shown that a variant of GBS was responsible for over 80% of meningitis cases in newborns. This variant expresses specific proteins on its surface, which play an essential role in crossing the blood-brain barrier that separates the blood from the brain.
Using complementary approaches, the researchers demonstrated that one of the proteins exclusively expressed by this variant specifically recognized two receptors present in the cerebral blood vessels that constitute the main element of the blood-brain barrier. Thanks to human samples, they have shown that these receptors are overexpressed in newborns. However, these brain receptors are not present in adults, which explains why GBS is only very rarely responsible for meningitis beyond the first year of life, given that the bacteria cannot reach the brain.
For the researchers, these findings open up interesting therapeutic research avenues, particularly in regard to meningitis treatments. “The idea would be to develop treatments to target these receptors in the blood-brain barrier. In the longer term, we would like to study the individual factors of susceptibility leading to the development of these infections. This would make it possible to perform personalized monitoring of at-risk infants born to mothers colonized by this variant,” explains Guignot.
[1] Physiological barrier between the blood and the brain that protects the latter from toxic substances and pathogenic microorganisms
[2]The Structural Molecular Biology and Infectious Processes laboratory (CNRS/Institut Pasteur), the Center for Interdisciplinary Research in Biology, (CNRS/Collège de France/Inserm), the Institute for Advanced Biosciences (CNRS/Inserm/UGA), among others, also participated in this research
Julie Guignot
Institut Cochin
E-mail : whyvr.thvtabg@vafrez.se
Téléphone : 01 40 51 64 13
CC17 Group B Streptococcus exploits integrins for neonatal meningitis development
Romain Deshayes de Cambrone1, Agnès Fouet1, Amandine Picart1, Anne-Sophie Bourrel1,2, Cyril Anjou1, Guillaume Bouvier4, Cristina Candeias1, Abdelouhab Bouaboud1, Lionel Costa1, Anne-Cécile Boulay5, Martine Cohen-Salmon5, Isabelle Plu6, Caroline Rambaud7, Eva Faurobert8, Corinne Albigès-Rizo8, Asmaa Tazi1,2,3, Claire Poyart1,2,3, Julie Guignot1*
1Université de Paris, Institut Cochin, INSERM, U1016, CNRS, UMR8104, F-75014 Paris, France
2 Hôpitaux Universitaires Paris Centre, Cochin, Assistance Publique Hôpitaux de Paris France;
3 Centre National de Référence des Streptocoques, France;
4 Structural Bioinformatics 10 Unit, Department of Structural Biology and Chemistry, Institut Pasteur, CNRS UMR3528, C3BI, 11 USR3756 Paris, France;
5 Center for Interdisciplinary Research in Biology (CIRB), Collège de France, CNRS UMR7241, INSERM U1050, PSL Research University, Paris, France;
6 Sorbonne Université /Département de neuropathologie Raymond Escourolle – Hôpital Pitié- Salpêtrière – Assistance Publique-Hôpitaux de Paris, France;
7 Université de Versailles Saint 15 Quentin en Yvelines (Université Paris-Saclay)/Service d’anatomie-pathologique et médecine 16 légale, Hôpital Raymond Poincaré, Garches, France;
8 INSERM U1209, CNRS UMR 5309, 17 Institute for Advanced Biosciences, France/Université Grenoble Alpes, F-38700 La Tronche, France.
Journal of Clinical Investigation, février 2021
DOI : 10.1172/JCI136737