Researcher Contact
Caroline Rouaux
Inserm researcher JRU-S 1329
Strasbourg Biomedical Research Centre
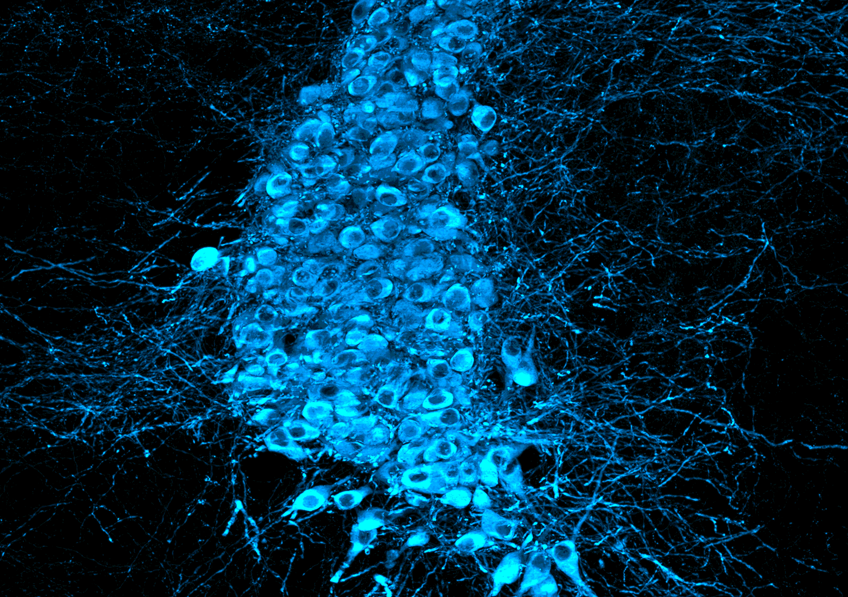 Noradrenergic neurons in the mouse locus coeruleus whose dysfunction contributes to cortical hyperexcitability in ALS. © Caroline Rouaux
Noradrenergic neurons in the mouse locus coeruleus whose dysfunction contributes to cortical hyperexcitability in ALS. © Caroline Rouaux
Amyotrophic lateral sclerosis or Charcot’s disease is a neurodegenerative disease that results in progressive paralysis and subsequent death. Diagnosing it is difficult and no curative treatment exists to date, making these challenges for research. In a new study, Inserm researcher Caroline Rouaux and her team at the Strasbourg Biomedical Research Centre (Inserm-Université de Strasbourg), in collaboration with researchers from Ludwig Maximilian University in Munich, CNRS and Sorbonne Université, show that electroencephalography could become a diagnostic and prognostic tool for the disease. Thanks to this type of examination, the scientists were able to reveal an atypical brain wave profile that could prove to be specific to the disease. Through this research, published in Science Translational Medicine, a potential therapeutic target has also been discovered. Fundamental advances that could ultimately benefit patients.
Amyotrophic lateral sclerosis (ALS), otherwise known as Charcot’s disease, remains a veritable challenge for clinicians. This neurodegenerative disease, which most often develops between the ages of 50 and 70, leads to progressive paralysis and death within just two to five years. It is caused by the death of the motor neurons – the nerve cells that control the muscles, both in the brain (central motor neurons) and in the spinal cord (peripheral motor neurons).
Diagnosing ALS is difficult because its initial signs vary from person to person: weakness or cramps in an arm or leg, trouble swallowing or slurred speech, etc. In addition, there is no biomarker specific to the disease. Therefore it is diagnosed by ruling out other conditions that can lead to motor disorders, which usually takes one to two years after the onset of symptoms, delaying the deployment of therapeutic measures and reducing the chances of inclusion in clinical trials at an early stage.
It was with the aim of shortening this time frame that Caroline Rouaux’s team at the Strasbourg Biomedical Research Centre, in collaboration with the teams of Sabine Liebscher in Munich and Véronique Marchand-Pauvert, Inserm researcher in Paris, tested the use of electroencephalography1. This inexpensive and easy-to-use technique involves placing electrodes on the surface of the skull to record brain activity in the form of waves.
The examination performed in subjects with ALS and in corresponding animal models revealed an imbalance between two types of waves associated with excitatory and inhibitory neuron activity, respectively. This imbalance, in favour of greater excitatory neuron activity to the detriment of inhibitory neurons, reflects cortical hyperexcitability.
‘This phenomenon is no surprise and had already been described with other investigation methods, but these are rarely used due to being difficult to implement and only work at the very beginning of the disease. Electroencephalography, however, is minimally invasive, very inexpensive, and can be used at different times during the disease. In addition, the atypical brain wave profile revealed by electroencephalography could prove to be specific to the disease,’ explains Rouaux, Inserm researcher and last author of the study.
Indeed, analysis of the electroencephalographic recording of the brain’s electrical activity reveals various types of brain waves of differing amplitudes and frequencies. One of these, called the theta wave, reflects the activity of the excitatory neurons that transmit messages stimulating neurons, while another wave, gamma, reflects that of the inhibitory neurons that block the transmission of nerve messages.
The study reveals that in humans and animals with ALS, the interaction between these two wave types is atypical, revealing an imbalance between the excitatory and inhibitory activities. Not only was this imbalance found in all the subjects tested, but the scientists also showed that the more the symptoms of the disease progress, the greater the imbalance. In addition, this atypical wave pattern was detected in animals even before the onset of the first motor symptoms.
If these initial findings are confirmed, electroencephalography could in the future serve as a prognostic tool for already-diagnosed patients in order to evaluate, for example, the response to a medication, or even as a diagnostic tool in the event of symptoms suggestive of the disease.
In the second part of this research, the scientists were able to study, in patients and mice, the mechanisms behind the hyperexcitability observed. First, they measured the levels of the different neuromodulators produced by the neurons to communicate with each other, and found a deficiency in one of them: noradrenaline was present in smaller amounts in the brains of the patients and mice with ALS compared to healthy brains.
To verify the role of noradrenaline, they blocked the production of this neuromodulator in healthy animals, and showed that doing so causes cortical hyperexcitability, such as that observed in the disease. And conversely, by administering molecules that stimulate the action of noradrenaline in a mouse model of ALS, the scientists reduced the hyperexcitability and restored brain activity equivalent to that of healthy mice.
‘This discovery could mark the opening of a new therapeutic avenue in ALS provided that cortical hyperexcitability is indeed associated with disease progression. Indeed, while we have seen an association between the two in our study, no causal link has been established for the moment. This is what we will be checking in the coming months.’ concludes Rouaux.
1 Electroencephalography is commonly used for research purposes in neurology but also in clinical practice. The examination provides information on brain activity in the event of sleep disorders, after a stroke, or even in the case of coma. It can also be used to diagnose encephalitis, epilepsy or confirm brain death.
Caroline Rouaux
Inserm researcher JRU-S 1329
Strasbourg Biomedical Research Centre
Cortical hyperexcitability in mouse models and patients with amyotrophic lateral sclerosis is linked to noradrenaline deficiency
Science Translational Medicine, 13 March 2024
www.science.org/doi/10.1126/scitranslmed.adg3665
Jelena Scekic-Zahirovic 1†‡, Cristina Benetton 2†, Aurore Brunet 1†, XiaoQian Ye 3,4, Evgeny Logunov3,4, Vincent Douchamps 5, Salim Megat1, Virginie Andry6, Vanessa Wing Yin Kan3,4, Geoffrey Stuart-Lopez1, Johan Gilet1, Simon J. Guillot 1, Sylvie Dirrig-Grosch1, Charlotte Gorin1, Margaux Trombini1, Stephane Dieterle1, Jerome Sinniger 1, Mathieu Fischer 1, Frederique Rene1, Zeynep Gunes 3,4, Pascal Kessler 7, Luc Dupuis1, Pierre-Francois Pradat2,8, Yannick Goumon 6, Romain Goutagny 5, Veronique Marchand-Pauvert 2§, Sabine Liebscher 3,4,9,10§, Caroline Rouaux 1§
1 Université de Strasbourg, Inserm UMRS 1329, Strasbourg Translational Neuroscience and Psychiatry (STEP), Centre de Recherche en Biomédecine de Strasbourg, 1 rue Eugene Boeckel, 67
000 Strasbourg, France.
2 Sorbonne Université, Inserm, CNRS, Laboratoire d’Imagerie Biomedicale, LIB, 75006 Paris, France.
3 Institute of Clinical Neuroimmunology, Klinikum der Universitat Munchen, Ludwig–Maximilians University Munich, 82152 Martinsried, Germany.
4 Biomedical Center, Ludwig–Maximilians University Munich, 82152 Martinsried, Germany.
5 Laboratoire de Neurosciences Cognitives et Adaptatives (LNCA), Université de Strasbourg, Faculté de Psychologie, 12 rue Goethe, 67000 Strasbourg, France; LNCA, UMR 7364, CNRS, 12 rue Goethe, 67000 Strasbourg, France.
6 CNRS UPR3212, SMPMS-INCI, Mass Spectrometry Facilities, Institut des Neurosciences Cellulaires et Intégratives, Centre National de la Recherche Scientifique and University of Strasbourg, 67 000 Strasbourg, France.
7 Inserm UMS 38, Centre de Recherche en Biomédecine de Strasbourg, Faculté de Médecine, Université de Strasbourg, 67000 Strasbourg, France.
8 Neurologie, AP-HP, Hôpital Pitié-Salpêtrière, 75013-Paris, France.
9 Munich Cluster for Systems Neurology (SyNergy), 81377 Munich, Germany.
10 Medical Faculty and Department of Neurology, University Hospital of Cologne, Cologne, 50937 Germany.
†These authors contributed equally to this work.
‡Present address: German Center for Neurodegenerative Diseases (DZNE), Ulm, Germany.
These authors jointly supervised this work.