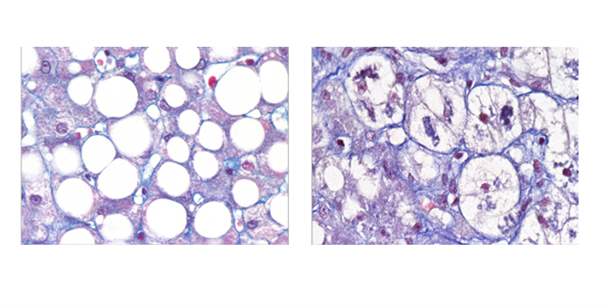
Data-driven cluster analysis identifies distinct types of metabolic dysfunctionassociated steatotic liver disease
Violeta Raverdy 1,2,24, Federica Tavaglione 3,4,24, Estelle Chatelain5,24, Guillaume Lassailly6,24, Antonio De Vincentis7,8, Umberto Vespasiani-Gentilucci3,4, Sami F. Qadri 9,10, Robert Caiazzo1,2, Helene Verkindt1,2, Chiara Saponaro1 , Julie Kerr-Conte1 , Gregory Baud1,2, Camille Marciniak1,2, Mikael Chetboun1,2, Naima Oukhouya-Daoud1,2, Samuel Blanck11, Jimmy Vandel 5 , Lisa Olsson 12, Rima Chakaroun12, Viviane Gnemmi13,14, Emmanuelle Leteurtre13,14, Philippe Lefebvre15, Joel T. Haas 15, Hannele Yki-Järvinen9,10, Sven Francque 16,17, Bart Staels 15, Carel W. Le Roux 18, Valentina Tremaroli 12, Philippe Mathurin6 , Guillemette Marot11,19, Stefano Romeo 12,20,21,22,23 & François Pattou 1,2
1 Translational Research for Diabetes UMR 1190, University of Lille, Inserm, Institut Pasteur Lille, CHU Lille, Lille, France.
2 Department of General and Endocrine Surgery, Centre Hospitalier et Universitaire de Lille, Lille, France.
3 Operative Unit of Clinical Medicine and Hepatology, Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy.
4 Research Unit of Clinical Medicine and Hepatology, Department of Medicine and Surgery, Università Campus Bio-Medico di Roma, Rome, Italy.
5 US 41 – UAR 2014 – PLBS Bilille, University of Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, F-59000, Lille, France.
6 Department of Hepato-Gastroenterology CHU Lille, University of Lille, Inserm INFINITE-U1286, Lille, France.
7 Operative Unit of Internal Medicine, Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy.
8 Research Unit of Internal Medicine, Department of Medicine and Surgery, Università Campus Bio-Medico di Roma, Rome, Italy.
9 Department of Medicine, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
10 Minerva Foundation Institute for Medical Research, Helsinki, Finland.
11 ULR 2694 METRICS: Évaluation des technologies de santé et des pratiques médicales, University of Lille, CHU Lille, F-59000, Lille, France.
12 Wallenberg Laboratory, Department of Molecular and Clinical Medicine, Institute of Medicine, University of Gothenburg, Gothenburg, Sweden.
13 Cancer Heterogeneity Plasticity and Resistance to Therapies, CANTHER-UMR9020-U1277 – CNRS, Inserm, CHU Lille, University of Lille, Lille, France.
14 Department of Pathology, CHU Lille, University of Lille, Lille, France.
15 Nuclear Receptors, Metabolic and Cardiovascular Diseases – U1011, University of Lille, Inserm, CHU Lille, Institut Pasteur Lille, Lille, France.
16 Department of Gastroenterology Hepatology, Antwerp University Hospital, Edegem, Belgium.
17 InflaMed Centre of Excellence, Laboratory for Experimental Medicine and Paediatrics, Translational Sciences in Inflammation and Immunology, Faculty of Medicine and Health Sciences, University of Antwerp, Wilrijk, Belgium.
18 Diabetes Complications Research Centre, University College Dublin, Dublin, Ireland.
19 MODAL: Models for Data Analysis and Learning, Inria, F-59000, Lille, France.
20 Clinical Nutrition Unit, Department of Medical and Surgical Sciences, University Magna Graecia, Catanzaro, Italy.
21 Department of Cardiology, Sahlgrenska University Hospital, Gothenburg, Sweden.
22 Department of Medicine Huddinge (H7), Karolinska Institutet and University Hospital, Stockholm, Sweden.
23 Department of Molecular and Clinical Medicine, Institute of Medicine, Gothenburg University, Gothenburg, Sweden.
24 These authors contributed equally: Violeta Raverdy, Federica Tavaglione, Estelle Chatelain, Guillaume Lassailly.
*This research was supported by the ‘Programme d’Investissement d’Avenir’ (PRECINASH, ANR-16-RHUS-0006; European Genomic Institute for Diabetes, ANR-10-LABX-0046), Lille University (WILL-CHAlRES-23-001), Fondation de la Recherche Médicale (EQU202303016330 PATTOU), EU Horizon 2020 research and innovation program (Innovative Medicines Initiative 2, project SOPHIA 875534).
Nature Medicine, décembre 2024
DOI : 10.1038/s41591-024-03283-1