Researcher Contact
Wolf Hervé Fridman
Unité Inserm 1138
Centre de recherche des Cordeliers
Tél : + 33 (0)6 89 98 22 65 rf.ueissuj.crc@namdirf.evrehWolf Hervé Fridman
Unité Inserm 1138 Centre de recherche des Cordeliers
Tél : + 33 (0)6 89 98 22 65
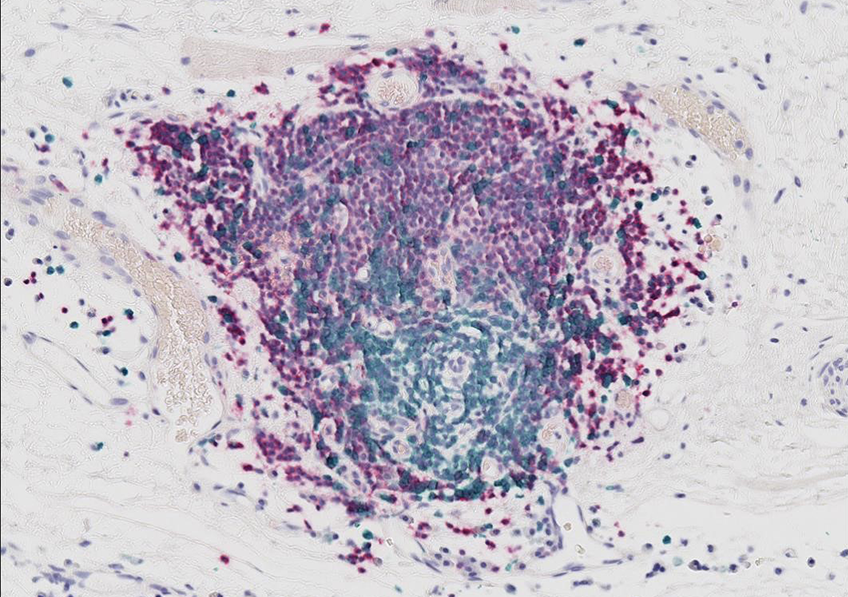
Tertiary lymphoid structures are cellular aggregates that contain many B-cells (in purple) located near tumors. This is the area where the antitumor immune response starts. ©Antoine Bougouin/Centre de recherche des Cordelier/Inserm, Sorbonne Université, Université de Paris
How can we improve and better personalize the treatment of soft tissue sarcomas, these particularly resistant and aggressive forms of cancer? An international team led by Wolf Hervé Fridman with researchers from Inserm, Sorbonne Université and Université de Paris at the Cordeliers Research Center, in collaboration with the French League against cancer and Institut Bergonié, has shown that B cells also play a major role in predicting of patient’s response to immunotherapy. It was previously thought only T cells could be used in this way. Their findings, to be published in Nature, pave the way for the personalization of treatments for patients with soft tissue sarcomas.
Soft tissue sarcomas are a heterogenous group of aggressive, chemotherapy-resistant cancers that affect the soft tissues of the body (fat, muscles, fibrous tissue, blood and lymphatic vessels, nerves, etc.). In the current clinical trials, only 15% of patients respond to immunotherapy, which raises the question of the needless exposure of the other patients to the toxicity of these treatments. Identifying markers that predict their response to immunotherapy is therefore crucial. A strategy that until now has been essentially focused on the T cells – immune cells capable of recognizing cells that are infected, cancerous or foreign to the body.
Through research published in Nature, a group led by Wolf Hervé Fridman with members from Inserm, Sorbonne Université and Université de Paris at the Cordeliers Research Center, in collaboration with the “Tumor identity card” team from the French League against cancer, Institut Bergonié, and teams from the USA and Taiwan, studied the question of identifying other potential markers.
They analyzed 608 tumors, classifying them into three groups according to the composition of their microenvironment[1]: immunologically poor tumors (low in immune cells and poorly vascularized), highly vascularized tumors, and immunologically rich tumors. The latter present aggregates of various cell types with high levels of B cells, the immune cells responsible for the production of antibodies. These aggregates are called tertiary lymphoid structures. The researchers observed that an anti-tumor immune response initiates within them, thereby showing that the B cells could play an anti-tumor role.
What is more, in a phase 2 clinical trial, the patients with immunologically rich tumors showed a high response rate (50%) to one immunotherapy: pembrolizumab. These patients also had a higher survival rate than those with immunologically poor or highly vascularized tumors.
A second study by a US team, co-signed by Wolf Hervé Fridman’s team at Cordeliers Research Center (Inserm/Sorbonne Université/Université de Paris), and published in parallel in Nature, extended these observations to include melanoma and kidney cancer.
The results of these studies show that in addition to the T cells that are usually researched, the B cells play an essential role in the response to immunotherapy for certain cancers. These cells bring new hope for the treatment of soft tissue sarcomas, which are particularly resistant to standard therapies. In addition, from a personalized medicine standpoint, these findings could help orient clinical decisions and patient treatment by means of a simple test to identify those whose tumors are immunologically rich.
On the basis of these results, an initial French clinical trial coordinated by Antoine Italiano (Institut Bergonié, Université de Bordeaux), co-author of the first article, and which includes patients with such tumors, is currently ongoing within the French Sarcoma Group.
[1] The tumor microenvironment corresponds to the biological elements that surround the tumor (blood vessels, immune cells, various types of cells, signaling molecules, extracellular matrix, etc.) and with which it interacts.
Wolf Hervé Fridman
Unité Inserm 1138
Centre de recherche des Cordeliers
Tél : + 33 (0)6 89 98 22 65 rf.ueissuj.crc@namdirf.evrehWolf Hervé Fridman
Unité Inserm 1138 Centre de recherche des Cordeliers
Tél : + 33 (0)6 89 98 22 65
B-cells and tertiary lymphoid structures contribute to immune checkpoint blockade response, Nature in press.
Beth A. Helmink1*, MD PhD; Sangeetha M. Reddy2*, MD MSci; Jianjun Gao3, MD PhD*; Shaojun Zhang4*, PhD.; Rafet Basar5, MD PhD; Rohit Thakur1, PhD; Keren Yizhak6, PhD; Moshe Sade-Feldman6,7, PhD; Jorge Blando8, DVM; Guangchun Han4; Vancheswaran Gopalakrishnan1, PhD; Yuanxin Xi10, PhD; Hao Zhao8, PhD; Wenbin Liu8; Valerie LeBleu9, PhD; Fernanda G. Kugeratsk9, PhD; Hussein A. Tawbi11, MD PhD; Rodabe N. Amaria11, MD; Sapna Patel11, MD; Michael A. Davies11, MD PhD; Patrick Hwu11, MD; Jeffrey E. Lee1, MD; Jeffrey E. Gershenwald1, MD; Anthony Lucci1, MD; Reetakshi Arora4, PhD; Scott Woodman11, MD PhD; Emily Z. Keung1, MD; Pierre-Olivier Gaudreau1, MD; Alexandre Reuben12, PhD; Christine N. Spencer13, PhD; Alex P. Cogdill1, MEng; Elizabeth M. Burton1, MBA; Lauren E. Haydu1, PhD; Alexander J. Lazar4,14,15, MD PhD; Roberta Zapassodi16, PhD; Courtney W. Hudgens14, BS; Deborah A. Ledesma14, PhD; SuFey Ong17, PhD; Michael Bailey17, PhD; Sarah Warren, PhD; Disha Rao17, MS; Oscar Krijgsman18, PhD; Elisa A. Rozeman18, MD; Daniel Peeper18, PhD; Christian U. Blank18, MD PhD; Ton N. Schumacher18, PhD; Lisa H. Butterfield19, PhD; Raghu Kalluri9, MD PhD; James Allison8, PhD; Florent Petitprez, PhD20,21,22; Wolf Herman Fridman, MD PhD20,21; Catherine Sautes-Fridman, PhD20,21; Nir Hacohen6,8, PhD; MD PhD; Katayoun Rezvani5,≠, MD PhD; Michael T. Tetzlaff14,15,≠, Padmanee Sharma3,8,≠, MD PhD; Linghua Wang4,≠, PhD; Jennifer A. Wargo1,4,≠, MD MMSc. 1 Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center 2 Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center 3 Department of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center 4 Department of Genomic Medicine, The University of Texas MD Anderson Cancer Center 5 Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center 6 Department of Medicine, Massachusetts General Hospital Cancer Center 7 Broad Institute of the Massachusetts Institute of Technology 8 Department of Immunology, The University of Texas MD Anderson Cancer Center 9 Department of Cancer Biology, The University of Texas MD Anderson Cancer Center 10 Department of Bioinformatics and Computational Biology, The University of Texas MD Anderson Cancer Center 11 Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center 12 Department of Thoracic / Head and Neck Medical Oncology, The University of Texas MD Anderson Cancer Center 13 Parker Institute for Cancer Immunotherapy 14 Department of Pathology, The University of Texas MD Anderson Cancer Center 15 Department of Translational and Molecular Pathology, The University of Texas MD Anderson Cancer Center 16 Immunology Program, Sloan Ketterin Institute, Memorial Sloan Kettering Cancer Center. 17 Nanostring Technologies, Seattle, WA 18 Division of Molecular Oncology and Immunology, The Netherlands Cancer Institute 19 Departments of Medicine, Surgery, Immunology and Clinical and Translational Science, University of Pittsburgh 20 INSERM, UMR_S 1138, Cordeliers Research Center, Team Cancer, immune control and escape, Paris, France 21 University Paris Descartes Paris 5, Sorbonne Paris Cite, UMR_S 1138, Centre de Recherche des Cordeliers, Paris, France 22 Programme Cartes d’Identité des Tumeurs, Ligue Nationale Contre le Cancer, Paris, France *Contributed equally ≠ Shared senior authorship
Nature : https://doi.org/10.1038/s41586-019-1922-8B-cells and tertiary lymphoid structures contribute to immune checkpoint blockade response, Nature in press. Beth A. Helmink1*, MD PhD; Sangeetha M. Reddy2*, MD MSci; Jianjun Gao3, MD PhD*; Shaojun Zhang4*, PhD.; Rafet Basar5, MD PhD; Rohit Thakur1, PhD; Keren Yizhak6, PhD; Moshe Sade-Feldman6,7, PhD; Jorge Blando8, DVM; Guangchun Han4; Vancheswaran Gopalakrishnan1, PhD; Yuanxin Xi10, PhD; Hao Zhao8, PhD; Wenbin Liu8; Valerie LeBleu9, PhD; Fernanda G. Kugeratsk9, PhD; Hussein A. Tawbi11, MD PhD; Rodabe N. Amaria11, MD; Sapna Patel11, MD; Michael A. Davies11, MD PhD; Patrick Hwu11, MD; Jeffrey E. Lee1, MD; Jeffrey E. Gershenwald1, MD; Anthony Lucci1, MD; Reetakshi Arora4, PhD; Scott Woodman11, MD PhD; Emily Z. Keung1, MD; Pierre-Olivier Gaudreau1, MD; Alexandre Reuben12, PhD; Christine N. Spencer13, PhD; Alex P. Cogdill1, MEng; Elizabeth M. Burton1, MBA; Lauren E. Haydu1, PhD; Alexander J. Lazar4,14,15, MD PhD; Roberta Zapassodi16, PhD; Courtney W. Hudgens14, BS; Deborah A. Ledesma14, PhD; SuFey Ong17, PhD; Michael Bailey17, PhD; Sarah Warren, PhD; Disha Rao17, MS; Oscar Krijgsman18, PhD; Elisa A. Rozeman18, MD; Daniel Peeper18, PhD; Christian U. Blank18, MD PhD; Ton N. Schumacher18, PhD; Lisa H. Butterfield19, PhD; Raghu Kalluri9, MD PhD; James Allison8, PhD; Florent Petitprez, PhD20,21,22; Wolf Herman Fridman, MD PhD20,21; Catherine Sautes-Fridman, PhD20,21; Nir Hacohen6,8, PhD; MD PhD; Katayoun Rezvani5,≠, MD PhD; Michael T. Tetzlaff14,15,≠, Padmanee Sharma3,8,≠, MD PhD; Linghua Wang4,≠, PhD; Jennifer A. Wargo1,4,≠, MD MMSc. 1 Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center 2 Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center 3 Department of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center 4 Department of Genomic Medicine, The University of Texas MD Anderson Cancer Center 5 Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center 6 Department of Medicine, Massachusetts General Hospital Cancer Center 7 Broad Institute of the Massachusetts Institute of Technology 8 Department of Immunology, The University of Texas MD Anderson Cancer Center 9 Department of Cancer Biology, The University of Texas MD Anderson Cancer Center 10 Department of Bioinformatics and Computational Biology, The University of Texas MD Anderson Cancer Center 11 Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center 12 Department of Thoracic / Head and Neck Medical Oncology, The University of Texas MD Anderson Cancer Center 13 Parker Institute for Cancer Immunotherapy 14 Department of Pathology, The University of Texas MD Anderson Cancer Center 15 Department of Translational and Molecular Pathology, The University of Texas MD Anderson Cancer Center 16 Immunology Program, Sloan Ketterin Institute, Memorial Sloan Kettering Cancer Center. 17 Nanostring Technologies, Seattle, WA 18 Division of Molecular Oncology and Immunology, The Netherlands Cancer Institute 19 Departments of Medicine, Surgery, Immunology and Clinical and Translational Science, University of Pittsburgh 20 INSERM, UMR_S 1138, Cordeliers Research Center, Team Cancer, immune control and escape, Paris, France 21 University Paris Descartes Paris 5, Sorbonne Paris Cite, UMR_S 1138, Centre de Recherche des Cordeliers, Paris, France 22 Programme Cartes d’Identité des Tumeurs, Ligue Nationale Contre le Cancer, Paris, France *Contributed equally ≠ Shared senior authorship Nature : https://doi.org/10.1038/s41586-019-1922-8