Researcher Contact
Nathalie Rouach
Chercheuse Inserm
U 1050 – Centre interdisciplinaire de recherche en biologie
(Inserm/CNRS/Collège de France)
angunyvr.ebhnpu@pbyyrtr-qr-senapr.se
Tél : 01 44 27 14 49
Téléphone portable sur demande
Primary culture of astrocytes © Inserm/Ruiz, Anne-Laure
Brain plasticity is a transient key period after birth in which the brain remodels the “wiring” of the neurons according to the external stimulations it receives (environment, interactions, etc.). The end – or “closure” – of this period marks the stabilization of the neural circuits, associated with efficient information processing and normal cognitive development. Plasticity is still possible in the future, although at a much lower level than at the beginning of life.
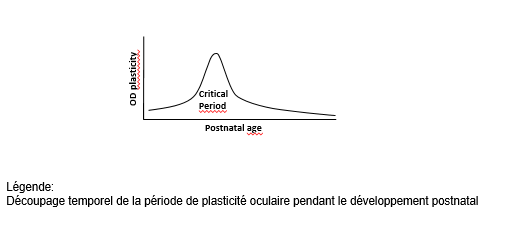
To remedy this, the researchers aim to remodel this wiring by identifying a therapy that would reintroduce brain plasticity, even once closure has occurred. To achieve this, they also seek to better characterize the biological mechanisms that underlie this closure.
Pioneering studies from the 1980s showed that transplanting immature astrocytes into the brains of adult animals reintroduced a period of major plasticity. The team of Inserm researcher and study coordinator Nathalie Rouach at the Center for Interdisciplinary Research in Biology (Inserm/CNRS/Collège de France)[1] took inspiration from this procedure to reveal the hitherto unknown cellular process responsible for the closure of plasticity.
Through experiments on the mouse visual cortex, the researchers show that the presence of immature astrocytes is the key to brain plasticity. The astrocytes are then later involved in developing interneuron maturation[1] during the plasticity period, ultimately leading to its closure. This maturation process occurs via a novel mechanism involving the protein Connexin 30, of which the researchers found high levels in mature astrocytes during closure.
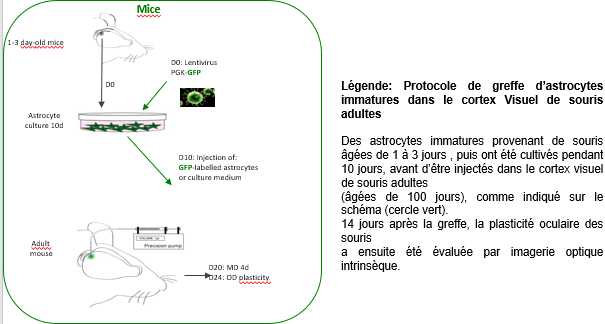
To find out, the researchers cultured immature astrocytes from the visual cortex of young mice (1 to 3 days’ old). These immature astrocytes were transplanted into the primary visual cortex of adult mice, following which the activity of the visual cortex was evaluated after four days of monocular occlusion – a standard technique used to assess brain plasticity. They found that the mice transplanted with the immature astrocytes presented a high level of plasticity, unlike the control mice which did not receive the transplant.
“This study is a reminder that in the neurosciences we must not only focus on neurons. The glial cells, of which the astrocytes are a subtype, regulate most of the brain’s functions. We realized that these cells have active roles. Glial cells are less fragile than neurons and so represent a more accessible means of acting on the brain, ” emphasizes Rouach.
[1] The interneurons establish connections between an afferent neural network (which sends information to the central nervous system) and an efferent neural network (which sends this information to the organs responding to the stimulation)
Nathalie Rouach
Chercheuse Inserm
U 1050 – Centre interdisciplinaire de recherche en biologie
(Inserm/CNRS/Collège de France)
angunyvr.ebhnpu@pbyyrtr-qr-senapr.se
Tél : 01 44 27 14 49
Téléphone portable sur demande
Astrocytes close the mouse critical period for visual plasticity
Science, juillet 2021
Jérôme Ribot1‡, Rachel Breton1,2,3‡#, Charles-Félix Calvo1 , Julien Moulard1,4 3 , Pascal Ezan1 , Jonathan Zapata1 , Kevin Samama1 , Matthieu Moreau5 , Alexis-Pierre Bemelmans6 4 , Valentin Sabatet7 , Florent Dingli7 , Damarys Loew7 , Chantal Milleret1 , Pierre Billuart5 , Glenn Dallérac1£#, Nathalie Rouach1£
1 Center for Interdisciplinary Research in Biology, Collège de France, CNRS UMR 7241, INSERM U1050, Labex Memolife, PSL Research University Paris, France
2 Doctoral School N°568, Paris Saclay University, PSL Research University, Le Kremlin Bicetre, France
3 Astrocytes & Cognition, Paris-Saclay Institute for Neurosciences, CNRS UMR 9197, Paris Saclay University, Orsay, France
4 Doctoral School N°158, Sorbonne University, Paris France
5 Université de Paris, Institute of Psychiatry and Neuroscience of Paris (IPNP), INSERM U1266, Genetic and Development of Cerebral Cortex Laboratory, GHU Paris Psychiatrie et Neurosciences, Hôpital Saint Anne, Paris, France
6 Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Département de la Recherche Fondamentale, Institut de biologie François Jacob, MIRCen, and CNRS UMR 9199, Université Paris-Saclay, Neurodegenerative Diseases Laboratory, Fontenay-aux-Roses, France
7 Institut Curie, PSL Research University, Mass Spectrometry and Proteomics Laboratory, Paris, France
‡ Equal contributing authors
£ Equal contributing authors