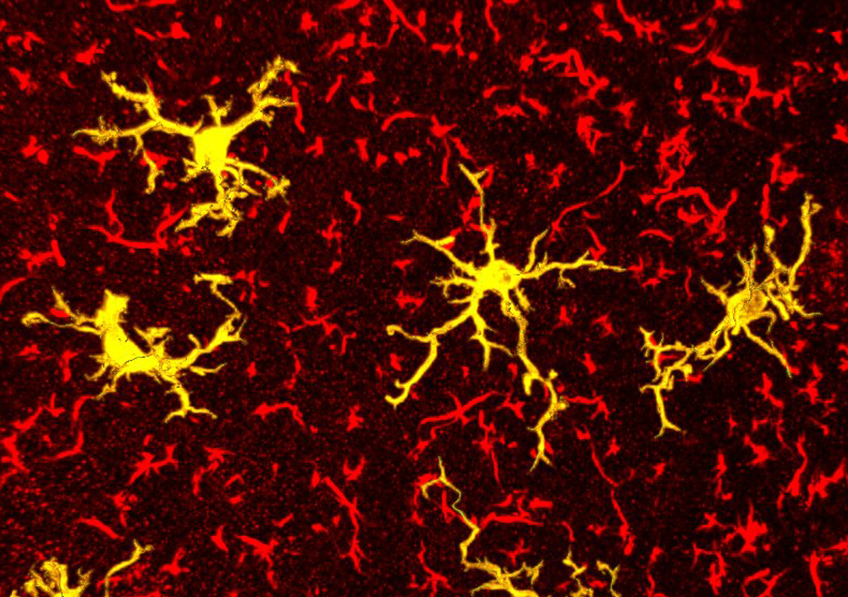
In yellow, microglia (immune cells of the brain) activated by the pro-inflammatory nature of a sunflower oil-enriched diet (fluorescence microscopy). © Clara Sanchez/Inserm
In yellow, microglia (immune cells of the brain) activated by the pro-inflammatory nature of a sunflower oil-enriched diet (fluorescence microscopy). © Clara Sanchez/Inserm
Obesity is a major public health problem, affecting around 650 million adults worldwide[1], and is often associated with systemic and cerebral inflammation as well as anxiety and cognitive disorders, such as memory deficits. In a new study, researchers from Inserm, CNRS and Université Côte d’Azur at the Institute of Molecular and Cellular Pharmacology tried to understand more precisely how diet can cause obesity, and its associated comorbidities. They focused more specifically on omega 6 (ω6) and omega 3 (ω3) fatty acids, exploring the health effects of various diets with different ratios of fatty acids (see box below). Their findings indicate that a diet enriched with ω6 (in this case, sunflower oil) is strongly associated with changes in metabolism, inflammation and cognitive functions, whereas one enriched with ω3 (in this case, rapeseed oil) has certain preventive effects. This research makes it possible to envisage dietary interventions based on a low ω6/ω3 ratio (thus with a preference for rapeseed oil over sunflower oil) to combat obesity and its associated neurological disorders. These findings have been published in Brain Behavior and Immunity.
According to the WHO, cases of obesity have almost tripled in number worldwide since 1975. Obesity is associated with numerous comorbidities (type 2 diabetes, cardiovascular diseases, osteoarthritis, cancer and cognitive disorders) and high mortality. While its causes are complex and involve the interaction of several factors, dietary imbalance is recognised as being the major contributing factor.
What is more, previous studies[1] have shown that obesity is associated not only with metabolic dysfunction, but also chronic inflammation in the peripheral organs (adipose tissues, liver, skeletal muscles and pancreas), as well as in the central nervous system (neuroinflammation). This neuroinflammation in obesity is characterised by an increase in pro-inflammatory markers in the region of the hypothalamus, an area of the brain known to control dietary behaviour[2]. However, the nature of the nutritional lipids that could be responsible for this neuroinflammation has not yet been elucidated.
In a new study, researchers from Inserm, CNRS and Université Côte d’Azur specifically focused on certain fatty acids that are essential for our bodies to function properly, and are known for having anti- and pro-inflammatory properties: omega 3 and 6 (see box below). Their objective was to better understand whether omega 3 and 6 are involved in the phenomenon of neuroinflammation in the context of a high-fat diet (so-called ‘obesogenic diet’), and whether they can be associated with the development of obesity.
Their research is also based on the observation of an increasingly strong trend in developed countries towards an excessive consumption of omega 6, whose inflammatory properties are abundantly documented in the scientific literature[4].
Omega 3 and omega 6: the importance of getting the right balance Omega 3 and omega 6 fatty acids are essential for the correct functioning of the body, which is unable to produce or synthesise them on its own. They therefore need to come from the diet and respect a certain balance (referred to as the omega 6/omega 3 ratio), in order to combine the pro-inflammatory properties of omega 6 with the anti-inflammatory properties of omega 3.
In animal models, the scientists evaluated the health effects of three obesogenic diets – high in lipids – each with a different fatty acids ratio.
In these diets, the researchers used vegetable oils that can be found in the shops, namely rapeseed (rich in omega 3) and sunflower (rich in omega 6). The first diet contained a high omega 6/omega 3 ratio, meaning that it was highly enriched in omega 6 and therefore in sunflower oil. The second had an intermediate ratio, with balanced levels of omega 3 and omega 6. And the third was highly enriched in omega 3 and therefore in rapeseed oil.
Through different examinations, the scientists measured the various effects of these diets on weight gain and fat storage, glucose homeostasis[5] response, development of anxiety and cognitive disorders, and brain inflammation.
At the end of the experiment, which lasted up to 5 months, the scientists observed (results summarised in the diagram below):
- altered metabolism, neuroinflammation and cognitive functions, including increased anxiety and spatial memory disorders in the obese mice fed a diet enriched with omega 6, and therefore sunflower oil,
- a protective effect of the omega 3-enriched, high-rapeseed oil diet, on weight gain, regulation of glucose homeostasis and the development of cognitive disorders.


‘While obesity was previously attributed to an increase in the inflammatory state, our study shows that such a state depends on the type of diet to which the animal is exposed. In other words, it is a diet high in omega 6 that is responsible for the inflammatory phenomena observed and not the obesity itself,’ explains Clara Sanchez, Inserm post-doctoral researcher and first author of the article.
‘This study also shows, for the first time, the protective effect against obesity and the associated inflammatory phenomena that a lipid-enriched diet can present, provided it promotes the consumption of omega 3. This research makes it possible to envisage dietary interventions based on a low ω6/ω3 ratio to combat obesity and its associated neurological disorders,’ explains Carole Rovère, Inserm researcher and last author of the article.
In their discovery, the scientists also observed in these mice a change in the shape of certain brain cells located in the hypothalamus – known as microglia – which appear to activate in response to a high-omega 6 diet. Their research will now focus on better understanding the specific role of these cells in obesity.
[2]Gregor and Hotamisligil, 2011; Thaler et al., 2012
[3] Baufeld et al., 2016; Cansell et al., 2021; De Souza et al., 2005; Le Thuc et Rovère, 2016; Salvi et al., 2022
[4] The WHO recommends consuming a ratio of omega 6 to omega 3 of 5:1. However, in Western societies our actual consumption is more like 15:1!
[5]Glucose homeostasis is a state of balance between the intake of glucose (intestinal absorption following a meal or production of glucose by the liver) and its use (glucose entry and use in the organs).