Maturation et persistance de la réponse lymphocytaire B mémoire anti-SARS-CoV-2
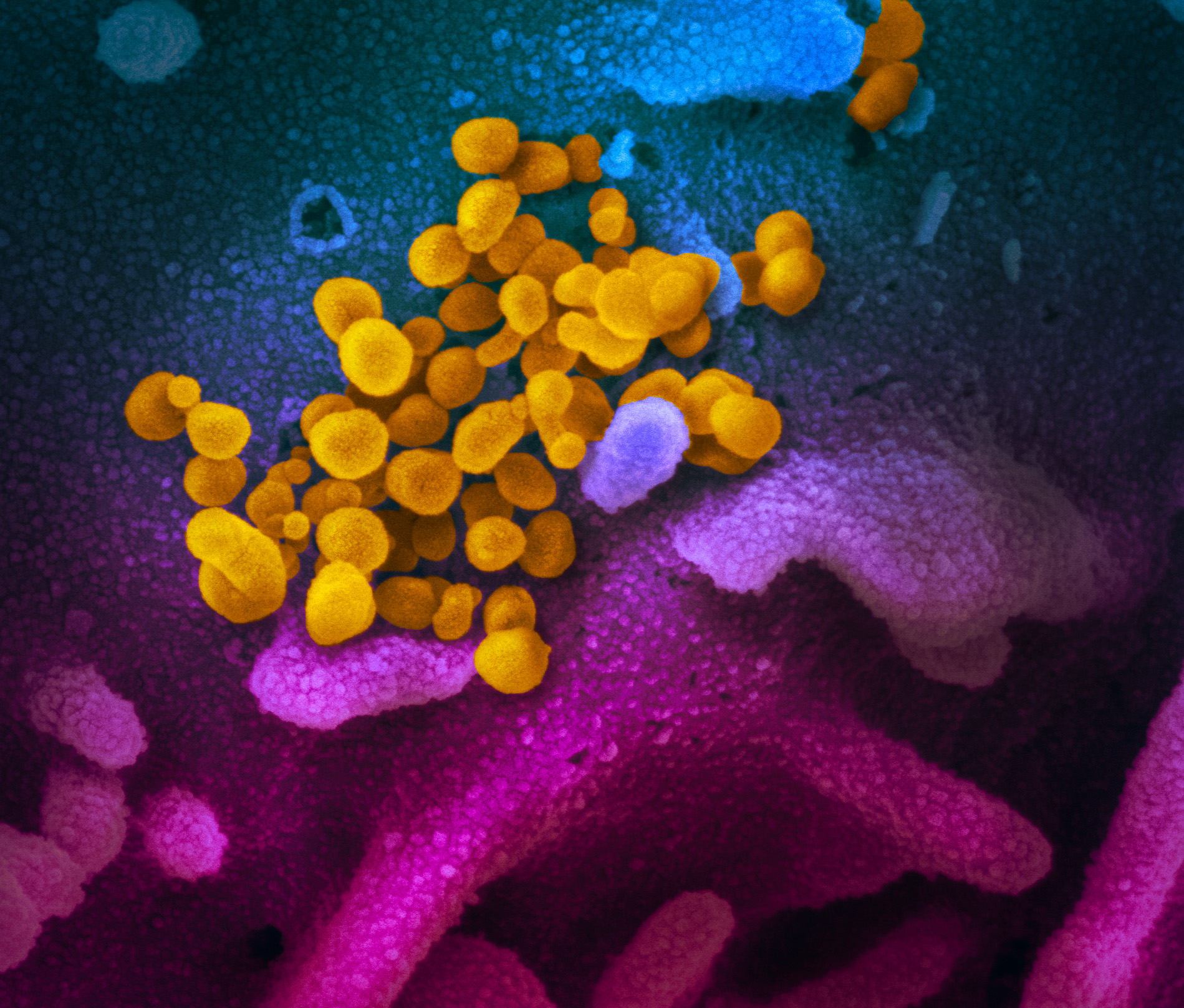

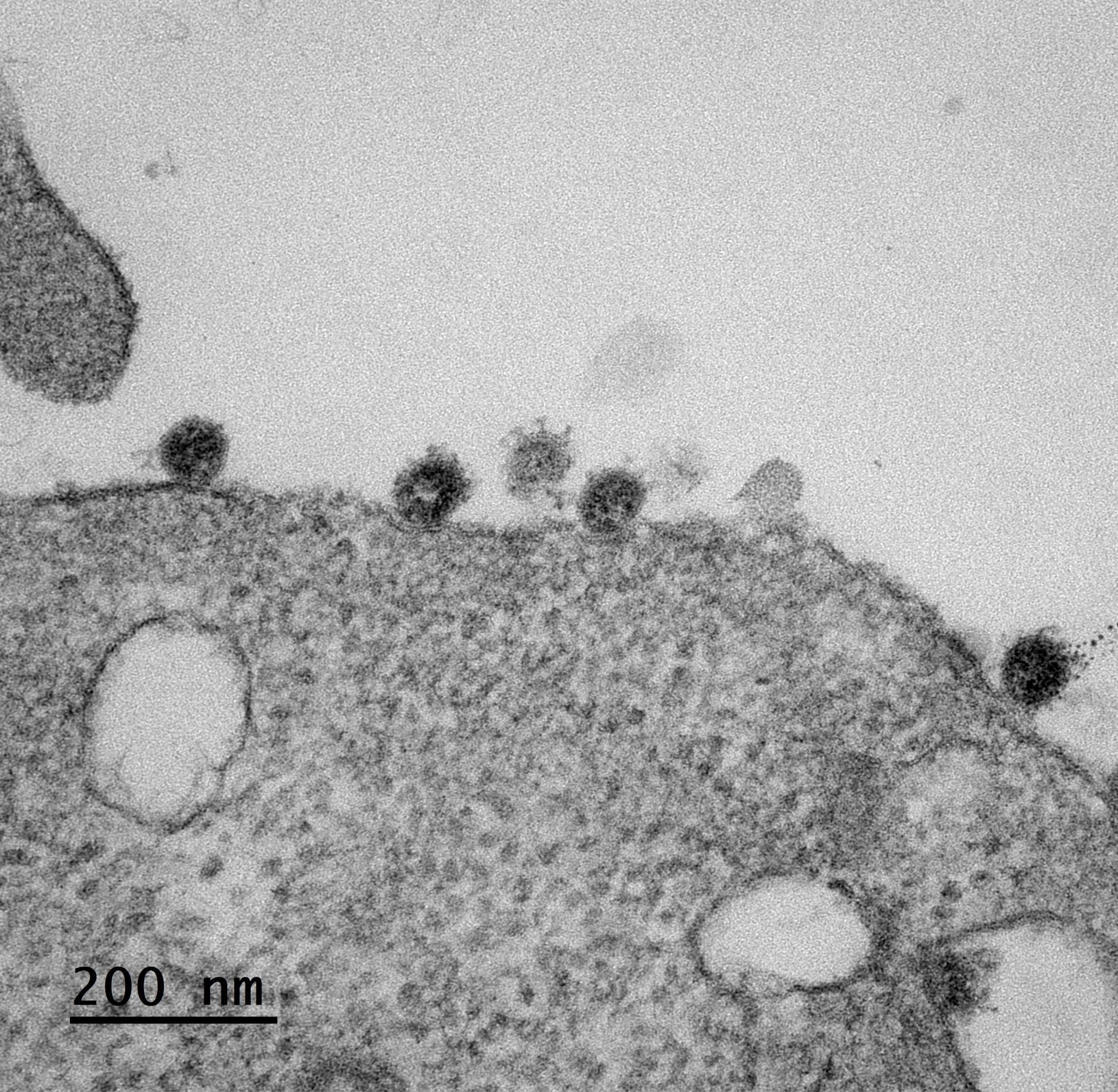
SARS-CoV-2 infected cell © Sébastien Eymieux and Philippe Roingeard, INSERM – Université de Tours
What are the factors predicting progression to severe forms of COVID-19? One year into the pandemic, this question remains a key research subject, and one that scientists from Inserm and Université de Paris decided to explore further by studying the link between viral kinetics and disease progression. This research is based on data from the Inserm-sponsored French COVID cohort, and has been published in PNAS.
While some patients infected with SARS-CoV-2 only have mild symptoms of COVID-19, a minority will go on to develop severe forms of the disease. A better understanding of the factors that determine this progression is essential if we are to improve their treatment and reduce mortality.
A team led by Inserm researcher Jérémie Guedj at the IAME laboratory (Inserm/Université de Paris) analyzed the biological data of 655 patients hospitalized for SARS-CoV-2 infection, and who were participants in the French COVID cohort.
Their study has highlighted two essential points. The first is that the older the patient, the longer he or she takes to eliminate the viral load from the nasopharyngeal compartment. The second is that this viral dynamic is associated with mortality.
While viral load is certainly not the only factor in progression to severe disease and death, it does play an important role. Although COVID-19 is often described as an inflammatory disease, these virological aspects must also be taken into account in the treatment and support of hospitalized patients.
As a consequence, this research also highlights the need for continued research into the development of antiviral treatments.

Injecting a vaccine with a pre-filled syringe. © Inserm/Depardieu, Michel
The Phase 3 clinical trial of a COVID-19 vaccine is to be launched via Covireivac, a platform set up under the auspices of Inserm and the university hospitals to centralize COVID-19 vaccine trials in France. Janssen, the pharmaceutical division of the Johnson & Johnson group, has obtained the authorizations[1] needed for ENSEMBLE 2, a trial evaluating the efficacy and safety of vaccine candidate Ad26.COV2.S in the prevention of COVID-19 in adults. In France, 1175 volunteers out of those registered on Covireivac will enroll in this clinical trial that will be conducted in 30,000 people across the world.
To conduct the French component of the trial, eight centers[2] have been selected to enroll the 1175 volunteers, representing around 147 per center. The frequency of the disease will be compared between those having received the vaccine and those having received placebo. The aim is to determine whether the administration of two doses of the study vaccine is effective against COVID-19 and whether the vaccine protects against SARS-CoV-2 infection and disease.
The candidate developed by Janssen is based on an attenuated version of a virus that causes rhinopharyngitis in humans (adenovirus) in order to:
This “non-replicating viral vector” vaccine is based on technology used in one of the Ebola vaccines, a product approved by the European Medicines Agency. It will be administered in the form of two intramuscular injections, with the second to be given 57 days (8 weeks) after the first. Details of the trial protocol are published on ClinicalTrials, a database of clinical studies conducted around the world.
The volunteers registered on Covireivac who have been selected to participate in this trial have already been contacted or will be shortly. To be eligible, the volunteers must, for example:
Ineligible for the trial are, for example, volunteers:
“Once a vaccine becomes available in France, it is legitimate that volunteers ask themselves whether they wish to participate in a trial in which some of them will receive placebo. For obvious ethical reasons, for those who will soon be able to access the national vaccination campaign, the answer is that these eventualities will be taken into account in the upcoming protocol amendments and that the option will be there to get vaccinated as part of that campaign should they so wish, even if they have already been enrolled in the trial.” declares Odile Launay, Scientific Manager of Covireivac, whose coordination team is based at Hôtel-Dieu Hospital – AP-HP.
At the request of MSS and MESRI, Inserm has, in coordination with the hospitals and general practitioners, been tasked with setting up infrastructure to conduct clinical trials on COVID-19 vaccines in France. Driven by Inserm, this platform, named Covireivac, federates 24 clinical investigation centers (CICs) located in university hospitals across France, in close collaboration with the College of teachers in general practice. The clinical operational aspects of the various university hospitals are coordinated by the Paris hospital group AP-HP.
Covireivac is based on I-REIVAC, the existing national network for clinical investigation in vaccinology, which has been reinforced and extended for the occasion. This network has been labeled a network of excellence by F-Crin (France’s national clinical research infrastructure). The platform’s infrastructure is funded by MSS and MESRI.
[1] From France’s drugs regulator (ANSM) and the Île-de-France institutional review board (CPP)
[2] 2 centers in the Île-de-France region (Cochin Hospital AP-HP and Saint Antoine Hospital AP-HP); 3 in Occitanie, 1 in Nouvelle Aquitaine, 1 in Auvergne Rhône Alpes and 1 in Grand Est.
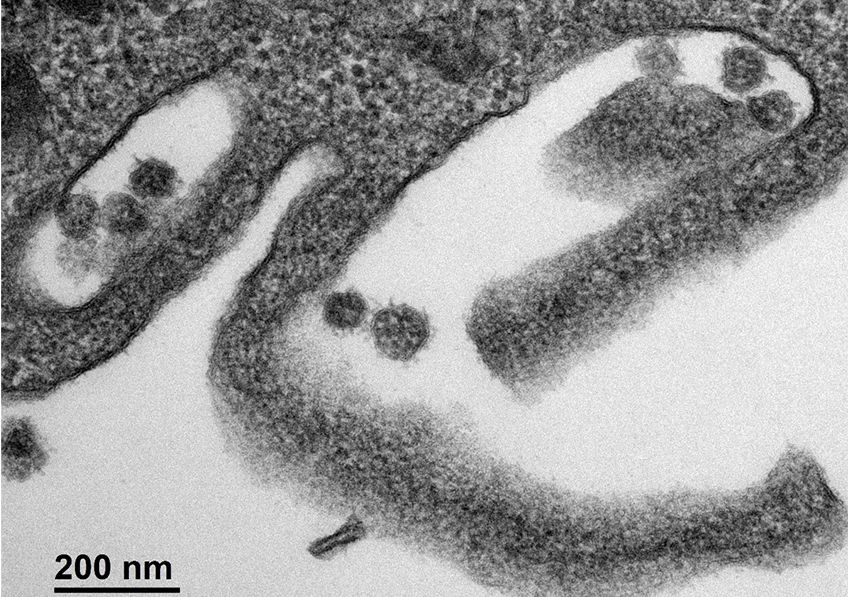

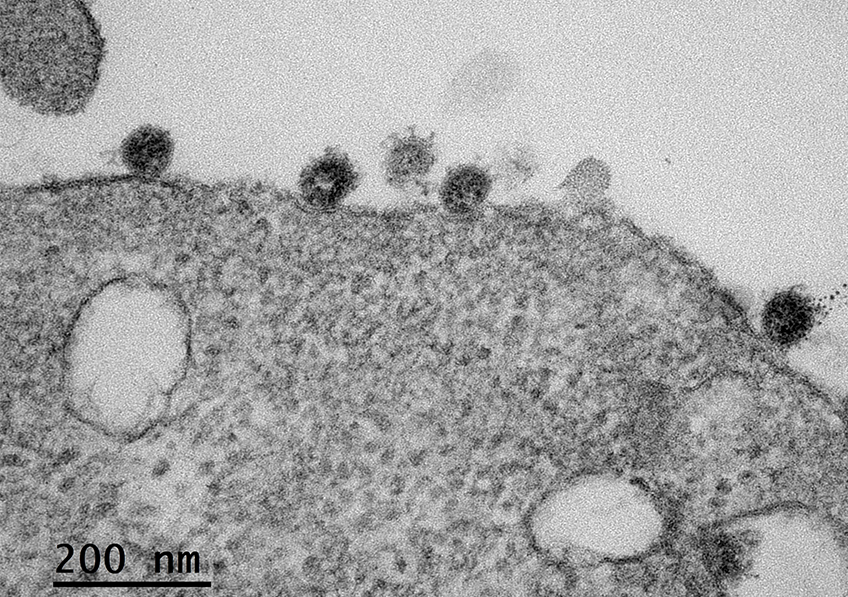
Cellule infectée par le SARS-CoV-2. © Sébastien Eymieux et Philippe Roingeard, INSERM – Université de Tours
As the COVID-19 pandemic continues, scientists are making significant headway in understanding the transmission of the SARS-CoV-2 coronavirus and the immune response it triggers at the time of infection. Researchers from Inserm, the Paris hospitals group AP-HP and Université de Paris, in collaboration with Rockefeller University in New York, have provided new data on the very early stages of immune response. Their findings have been published in Journal of Experimental Medicine.
Understanding the immune response against SARS-CoV-2 is essential if we are to know who is at risk of developing severe forms of COVID-19 and how to treat them effectively. While many studies have been conducted in patients in the advanced stages of infection, when they are already showing signs of severity, little is known about the very early stages of immune response against the virus.
Thanks to close collaboration between the Inserm teams of Ali Amara, virologist, and Vassili Soumelis, immunologist at Saint-Louis Research Institute (Université de Paris/Inserm/AP-HP), a study published in the Journal of Experimental Medicine was able to characterize innate immune response[1] within 24 to 48 hours following contact with the SARS-CoV-2 virus.
The researchers used immune cells called “plasmacytoid predendritic cells” as a model of innate immune cells that play an essential role in antiviral immunity by producing large amounts of interferon-alpha[2].
Analysis of this in vitro reconstituted response showed that SARS-CoV-2 induced effective and complete activation of the plasmacytoid predendritic cells. These then produced large amounts of interferon-alpha (the first line of defense against viruses) and differentiated into dendritic cells capable of activating T cells (which correspond to specific immunity cells). The researchers were also able to show that this activation of the plasmacytoid predendritic cells was partially inhibited by hydroxychloroquine, which would call for caution in the use of this molecule.
In the second part of the study, the teams collaborated with Jean-Laurent Casanova’s team from the Imagine Institute (Inserm/Université de Paris/AP-HP) and Rockefeller University in New York, in order to study the response of plasmacytoid predendritic cells from patients with genetic deficits for certain important genes of innate immunity. The objective was to clarify the molecular mechanisms involved in the response of these immune cells to SARS-CoV-2.
These experiments performed on samples obtained directly from patients showed that the response of plasmacytoid predendritic cells is dependent on UNC93B and IRAK-4, two important molecules of innate antiviral immunity. This research as a whole makes it possible to clarify early immune response to SARS-CoV-2 as well as some of its molecular determinants.
[1] Innate immunity is the body’s first line of defense and is triggered as soon as the body is exposed to a bacterium or virus (such as SARS-CoV-2). The innate immunity cells can contribute to the total destruction of the detected microbes or present them to the mechanisms of acquired immunity to facilitate their destruction by specific mechanisms (T and B cells).
[2] Interferons are cytokines (proteins) whose production is induced following viral, bacterial or parasitic infection, or the presence of tumor cells. While their main function is to interfere with viral replication, they also have an antibacterial and antiproliferative action, as well as an activating effect on other immune cells.

©
The Discovery trial was originally launched in March 2020 by Inserm to evaluate possible treatments for COVID-19. Its European expansion (Discovery Europe) was made possible by the EU-RESPONSE[1] project funded by the European Commission (see details in the box below). On January 13th, 2021, the Discovery Europe trial Data Safety Monitoring Boards (DSMB) evaluated an interim report based on 776 patients of whom 389 received remdesivir and 387 received standard of care. The efficacy of the treatment was evaluated after 15 days and measured on the WHO-7-point ordinal scale. As a result of the evaluation, the DSMB recommended that patient recruitment be suspended.
This recommendation was based on lack of evidence of efficacy of remdesivir after 15 days and a very low probability to conclude with the inclusion of additional participants. There was also no evidence for treatment efficacy at day 29 (on the same scale or on mortality), nor in the analysis restricted to moderate-risk participants at day 15. This recommendation has been endorsed by the Discovery Europe Steering Committee.
Discovery researchers are now collecting and monitoring data on all participants enrolled in the clinical study in order to publish their detailed scientific findings in a peer reviewed scientific journal.
The Discovery Europe trial will continue in 80 centres from 14 European countries and will soon launch the clinical evaluation of a combination of two monoclonal antibodies. Beside the deployment of vaccines, it remains paramount to provide strong evidence for adding effective medicines for the treatment of patients affected by Covid-19.
The Discovery trial was originally launched in March 2020 by Inserm to evaluate possible treatments for Covid-19. An agreement was signed with the WHO Solidarity trial so that it became an add-on trial of Solidarity. Discovery is now part of the EU-RESPONSE project (Discovery Europe), funded through Horizon 2020, the EU’s research and innovation programme. It is a multicentre adaptative randomized platform trial for the evaluation of the clinical and virological efficacy, as well as the safety, of candidate treatment versus standard of care in hospitalized adult patients with laboratory confirmed Covid-19. The initial set of tested treatments includes lopinavir/ritonavir, lopinavir/ritonavir plus IFN-b-1a, hydroxychloroquine, and remdesivir. The primary endpoint is the clinical status at day 15, measured on the WHO 7-point ordinal scale..
In June 2020, the DSMBs of Solidarity recommended to stop the hydroxychloroquine arm for futility concern as well as both lopinavir/ritonavir containing arms for futility and safety concern. In July 2020, continuing the evaluation of remdesivir, approved for conditional marketing authorisation in the European Union, was felt important because more data were needed to fully assess its efficacy.
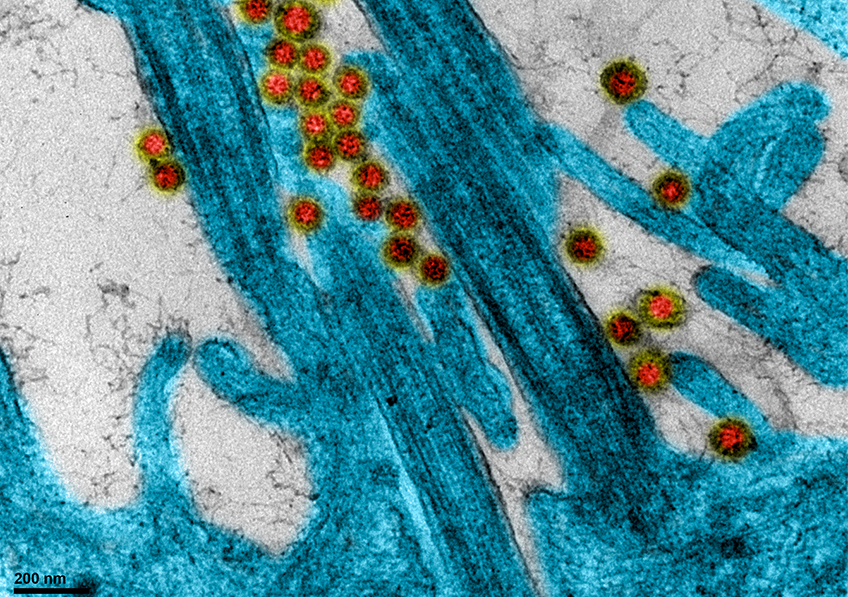

SARS-CoV-2 coronavirus attached to the cilia of human respiratory epithelial cells.© Manuel Rosa-Calatrava, INSERM ; Olivier Terrier, CNRS ; Andrés Pizzorno, Signia Therapeutics ; Elisabeth Errazuriz-Cerda UCBL1 CIQLE. VirPath (Centre International de Recherche en Infectiologie U1111 Inserm – UMR 5308 CNRS – ENS Lyon – UCBL1). Colorized par Noa Rosa C.
A study by Inserm and Dijon University Hospital based on French nationwide data on around 130,000 patients hospitalized for either COVID-19 or seasonal influenza shows that the mortality rate among those admitted for COVID is three times higher than that of seasonal influenza. These findings have been published in The Lancet Respiratory Medicine
This study uses data from the French national administrative database (medicalized information system program – PMSI), which contains information on all patients admitted to public and private hospitals in France, such as the reasons for their admission and the treatment they received. The researchers compared the admissions for COVID-19 (between March 1 and April 30, 2020) with those for seasonal influenza (between December 1, 2018 and February 28, 2019).
The results reveal:
The researchers point out that their study has several limitations. In particular, testing practices for influenza are likely to be highly variable across hospitals, whereas practices for COVID-19 may be more standardized. This may partly explain the increased number of admissions for COVID-19 compared to seasonal influenza. In addition, the difference in hospitalization rates may be partly due to existing immunity to influenza in the population, either from previous infection or vaccination.
“This study is the largest to date comparing the two diseases and confirms that COVID-19 is much more serious than influenza. The finding that the COVID-19 death rate was three times higher than that of seasonal influenza is particularly striking when one recalls that in the last five years the 2018/2019 influenza season has been the worst in France in terms of number of deaths,” declares Catherine Quantin, researcher at Inserm and Professor at Dijon University Hospital.
“Taken together, these findings clearly indicate that COVID-19 is much more severe than seasonal flu. While no treatment has yet been shown to be effective in preventing serious illness in COVID-19 patients, this study underlines the importance of the various prevention measures (barrier measures) and highlights the need for access to effective vaccines,” concludes Pascale Tubert-Bitter, research director at Inserm.
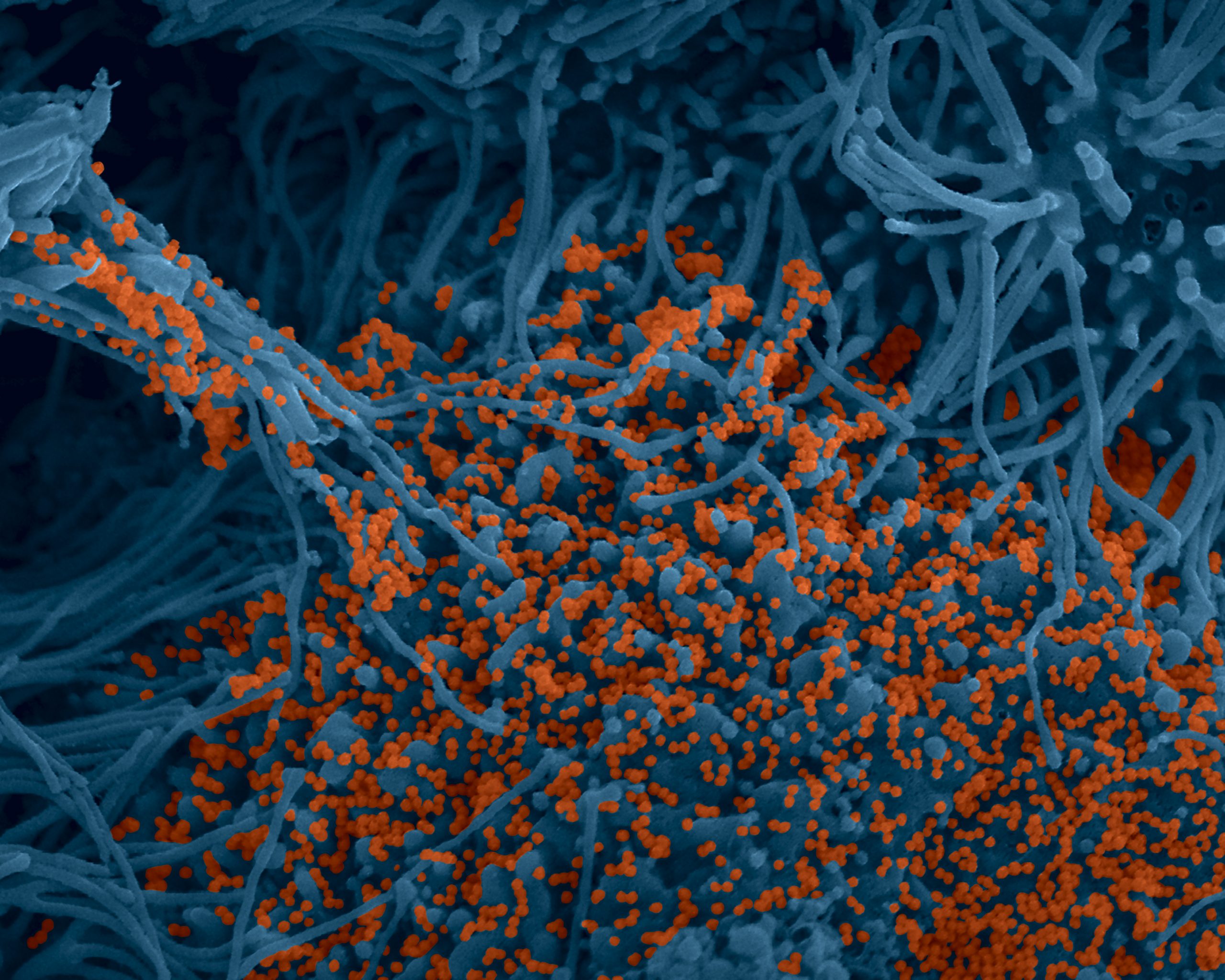

Colorized image of human bronchial cells (blue) infected with SARS-CoV-2 virus (orange). © Institut Pasteur. Image by Rémy Robinot, Mathieu Hubert, Vincent Michel, Olivier Schwartz & Lisa Chakrabarti, colors by Jean Marc Panaud
Teams from the Pitié-Salpêtrière AP-HP hospital, Sorbonne University, Inserm and the Pasteur Institute have carried out work to study the role that IgA-type antibodies play in the protection of body against Covid-19 in the mucous membranes, in particular respiratory. This work in press in Science Translational Medicine , and which is the subject of a pre-publication on Monday, December 7, 2020 on the website of the journal Science Translational Medicine , shows that the IgA antibody response plays a key role in neutralizing the early and particularly effective SARS-CoV-2 virus.
IgA-type antibodies play an essential role in the protection of the organism at the level of the mucous membranes, in particular respiratory. It therefore made sense to study this particular antibody response in patients infected with the SARS-CoV-2 virus. Researchers and clinicians affiliated with the CIMI Research Center (Sorbonne University and Inserm) in collaboration with several clinical departments of APHP-Sorbonne University and teams from the Institut Pasteur, show that the IgA response plays a key role in neutralizing the early and particularly effective SARS-CoV-2 virus.
Somewhat surprisingly, IgA antibodies are often even the first detectable virus specific antibodies. An unusual profile since immunological dogma wants the IgM response to be dominant when encountered with an unknown pathogen. The stimulation induced by the virus induces a very large expansion of young cells secreting IgA antibodies (plasmablasts) which circulate between the blood and the mucous membranes in which they are able to reside. However, this IgA antibody response is slowly declining, including in the saliva.
In conclusion, this work highlights the powerful protective nature of IgA. It raises the question of the possible role of secretory IgA in limiting the transmission of the virus.


©Adobe Stock
Back at the start of the pandemic, Inserm, through its REACTing consortium, set up Discovery: a European clinical trial to evaluate the efficacy of four antiviral drugs repurposed for the treatment of patients hospitalized with COVID-19 (remdesivir, hydroxychloroquine, lopinavir and interferon beta-1a). In parallel, the World Health Organization (WHO) set up Solidarity, a major consortium of clinical trials also aimed at testing the efficacy of these four treatments. Discovery then joined forces with Solidarity to help supply it with robust and rigorous data. The initial results of Solidarity have now been published in the New England Journal of Medicine.
Launched in March 2020 under the aegis of Solidarity – the World Health Organization (WHO) global clinical trials – Discovery is a clinical trial to study efficacy and safety. It is the only large-scale European academic trial of COVID-19 treatments.
Focusing on patients hospitalized with severe COVID-19 in France and other European countries, this trial is both randomized (the treatments are randomly assigned to the participants) and open-label (the patients and their caregivers know which treatment they have been allocated). The data obtained in Discovery form part of the data analyzed within the framework of Solidarity and have been presented in the study by the New England Journal of Medicine.
The analysis covers 11,330 adult patients in 405 hospitals across 30 countries and includes Solidarity’s “daughter trials” (with one of the main contributors being Discovery). The patients were assigned to different groups in order to receive one of the following regimens:
The results suggest that none of these treatments have an effect on the clinical improvement of patients. None of them significantly reduce overall mortality, the risk of having to initiate mechanical ventilation, or the duration of hospitalization.
However, within the framework of Solidarity and Discovery, it has nevertheless been decided to continue enrolments in the remdesivir group. Indeed, the meta-analysis presented in the study suggests that although mortality is not reduced in the mechanically ventilated patients in intensive care having received remdesivir, it does show that remdesivir may slightly reduce mortality in the subgroup of hospitalized patients who do not require mechanical ventilation. In addition, while a scientific consensus is emerging as to the lack of efficacy of the other therapeutic combinations tested in Solidarity, data published in other studies remain contradictory on the subject of remdesivir.
The decision by the Discovery investigators to continue enrolments in this group has therefore been made with the aim of obtaining new data in order to decide one way or the other on the utility of remdesivir in severe COVID-19.


Researchers have identified biomarkers in blood samples taken from diabetic patients. © Inserm/Latron, Patrice
Type 2 diabetes is a risk factor for the development of a severe form of Covid-19. Identifying the immune- and inflammatory markers associated with these severe forms of the disease in this patient population would enable earlier and more appropriate care. Researchers from Inserm, the Paris hospitals group AP-HP and Université de Paris have identified an immune signature in hospitalized diabetic patients that would make it possible to predict the risk of admission to intensive care. Their findings have been published in EMBO Molecular Medicine and supplement those of other studies published in recent months on the identification of biomarkers predictive of severe forms of Covid-19.
In the early months of the Covid-19 pandemic, type 2 diabetes was identified as a risk factor for developing a severe form of the disease and has been linked to higher mortality. Therefore, understanding why this is and identifying biomarkers to predict which diabetic patients will progress to a severe form of Covid-19 requiring intensive care constitutes a research priority in order to improve their care and increase their chances of survival.
As part of the team led by Inserm Research Director Nicolas Venteclef at Cordeliers Research Center (Inserm/Université de Paris/Sorbonne Université), researchers Fawaz Alzaid and Jean-Baptiste Julla prepared an observational study in a hospital setting. It was conducted at the University Center for the Study of Diabetes and its Complications led by Jean-François Gautier, a diabetologist researcher at Lariboisière Hospital AP-HP. The objective was to better understand the link between pre-existing inflammation in diabetes and the risk of developing a severe form of Covid-19. The scientists sought to characterize the immune and inflammatory “signatures” of diabetic patients hospitalized following infection with SARS-CoV-2 and who presented severe symptoms of the disease.
They looked at the immune response of 45 patients hospitalized with Covid-19, thirty of whom had type 2 diabetes. Among the study participants, 35% of the diabetic patients developed a severe form of the disease requiring a stay in intensive care, compared to 25% of the non-diabetic hospitalized patients.
The researchers analyzed blood samples from all of the patients. They found that those most severely affected had fewer lymphocytes (a type of white blood cell) than those who had not been in intensive care. The team observed particularly low levels of cytotoxic CD8+ lymphocytes, immune cells particularly involved in the antiviral response with important functions of recognizing and eliminating infected cells. This was observed in all of the intensive care patients, regardless of diabetic status.
However, the diabetic patients having required intensive care differed from non-diabetic patients in the same case because they also had fewer monocytes (another type of white blood cell) in their blood. Changes in the morphology of these monocytes were also observed, as these immune cells in patients with type 2 diabetes had a larger average size than those found in blood samples from non-diabetic patients.
Finally, the researchers noted an increased presence of inflammatory markers associated with the type 1 interferon pathway, powerful antiviral molecules.
“These findings have major clinical implications as they suggest that there is an immune- and inflammatory signature specific to diabetic patients at risk of developing severe Covid-19. If physicians notice a decrease in monocyte frequency and a change in their morphology, they have the possibility to identify patients who will require further follow-up and potentially a place in intensive care. This will make it possible to refine and improve care,” explains Inserm researcher Fawaz Alzaid.
This research also provides data to support ongoing clinical studies that suggest the importance of a disruption of the type 1 interferon pathway in the development of severe forms of the disease, and the potential therapeutic value of anti-interferon drugs, already highlighted in recent research involving Inserm, published in Science.

International research is being mobilized in order to develop safe and effective vaccines for COVID-19. Around thirty vaccine candidates are at the clinical evaluation stage, with some undergoing Phase 3 trials to demonstrate their efficacy. At the request of the Ministry of Solidarity and Health and the Ministry of Higher Education and Research, France – drawing on the excellence of its clinical research in vaccination – has taken steps to help evaluate the most promising vaccine candidates with the deployment of the COVIREIVAC platform. Driven by Inserm, COVIREIVAC federates 24 Clinical Investigation Centers (CICs) located in university hospitals across France, in close collaboration with the College of Teachers in General Practice. The clinical operational aspects of the various university hospitals are coordinated by the Paris Hospital Group AP-HP. Today, COVIREIVAC opens the registration process for volunteers to participate in the first large-scale clinical trials in France.
To make these trials possible, COVIREIVAC is looking for 25,000 volunteers aged 18 or over and has launched the registration and information website www.covireivac.fr. Developed with the support of Public Health France and the Medicines Agency (ANSM), it aims to provide the most accurate information possible on vaccine development so that potential volunteers can make an informed decision.
Volunteers in COVID-19 vaccine trials have a role to play in fighting the pandemic, moving research forward and thus contributing in the medium term to their own protection and that of their fellow citizens – particularly the most vulnerable. Becoming a volunteer also means participating in a scientific challenge alongside the scientific and medical community.
If you are interested in volunteering, simply pre-register at www.covireivac.fr and complete a preliminary health questionnaire. Volunteers will then be contacted according to the needs of the various trial protocols (age, pre-existing conditions, geographical location), following which they can either confirm or withdraw their agreement to participate in the specific trial for which they have been called. It is also possible that they may never be called.
Two vaccine clinical trials are currently ongoing in France: a Phase 1 trial in healthy subjects for a vaccine developed by Institut Pasteur in collaboration with CEPI, Themis and MSD which has begun at Cochin Hospital (Paris Hospital Group AP-HP), and a trial in healthcare workers on the contribution of the BCG vaccine to boosting systemic immunity and protection against COVID-19, which is coordinated by AP-HP.
Two types of large-scale clinical trial are envisaged in France. The first is Phase 2 trials to closely study the ability of vaccines to produce an immune response (immunogenicity) in elderly people, whose immune system is generally weakened despite being most at risk of developing severe forms of the disease. The second is Phase 3 trials for the large-scale study of the efficacy and safety of promising vaccine candidates, depending on the intensity of the virus’ circulation in France in the months to come.
These clinical trials could start between October and the end of the year, depending on the evolution of the epidemic and the ongoing discussions with industry.
“Good clinical trials are crucial for the development of safe and effective vaccines. As researchers and doctors, we are all committed to rigorous evaluation that will provide the health authorities with the essential data to guarantee the quality of the vaccines developed. What wenow need is volunteers to mobilize alongside us,” emphasizes Odile Launay, Professor of Infectious and Tropical Diseases at Université de Paris, coordinator of Cochin-Pasteur CIC at Cochin Hospital (AP-HP), and coordinator of COVIREIVAC.
In addition to the follow-up and monitoring of the volunteers during the trials, a specific system for monitoring participants will be set up by the platform at the end of the trials, in conjunction with primary care doctors and ANSM. This monitoring will therefore make it possible to track the safety of the vaccines over the long term.
The COVIREIVAC platform is working in close collaboration with the Scientific Committee for COVID-19 Vaccines, chaired by Inserm Research Director and CARE Committee member Marie-Paule Kieny. The clinical trials conducted will focus on the most promising vaccines, selected by the Scientific Committee.