© Pierre-Emmanuel Rautou, AP-HP Inserm
A trial sponsored by AP-HP and conducted by teams of Beaujon Hospital, AP-HP, Inserm and Université Paris Diderot was set up to establish an indicator that reflects the severity of liver disease in patients with cirrhosis. Coordinated by Professor Pierre-Emmanuel Rautou and Dr. Audrey Payancé the Hepatology Service of Beaujon Hospital, AP-HP, this study establishes that measurement microvesicles rate from liver and circulating in the blood greatly improves prediction of 6-month mortality of patients with cirrhosis.
This indicator would better choose treatments offer in patients with cirrhosis.These results, published in the journal Hepatology , also opening a reflection on the value of strategies to reduce the rates of these microvesicles in the blood of patients with cirrhosis.
Cirrhosis is the advanced stage of liver disease. It is estimated that the disease affects 200 000 to 500 000 in France and is responsible for 170,000 deaths per year in Europe.
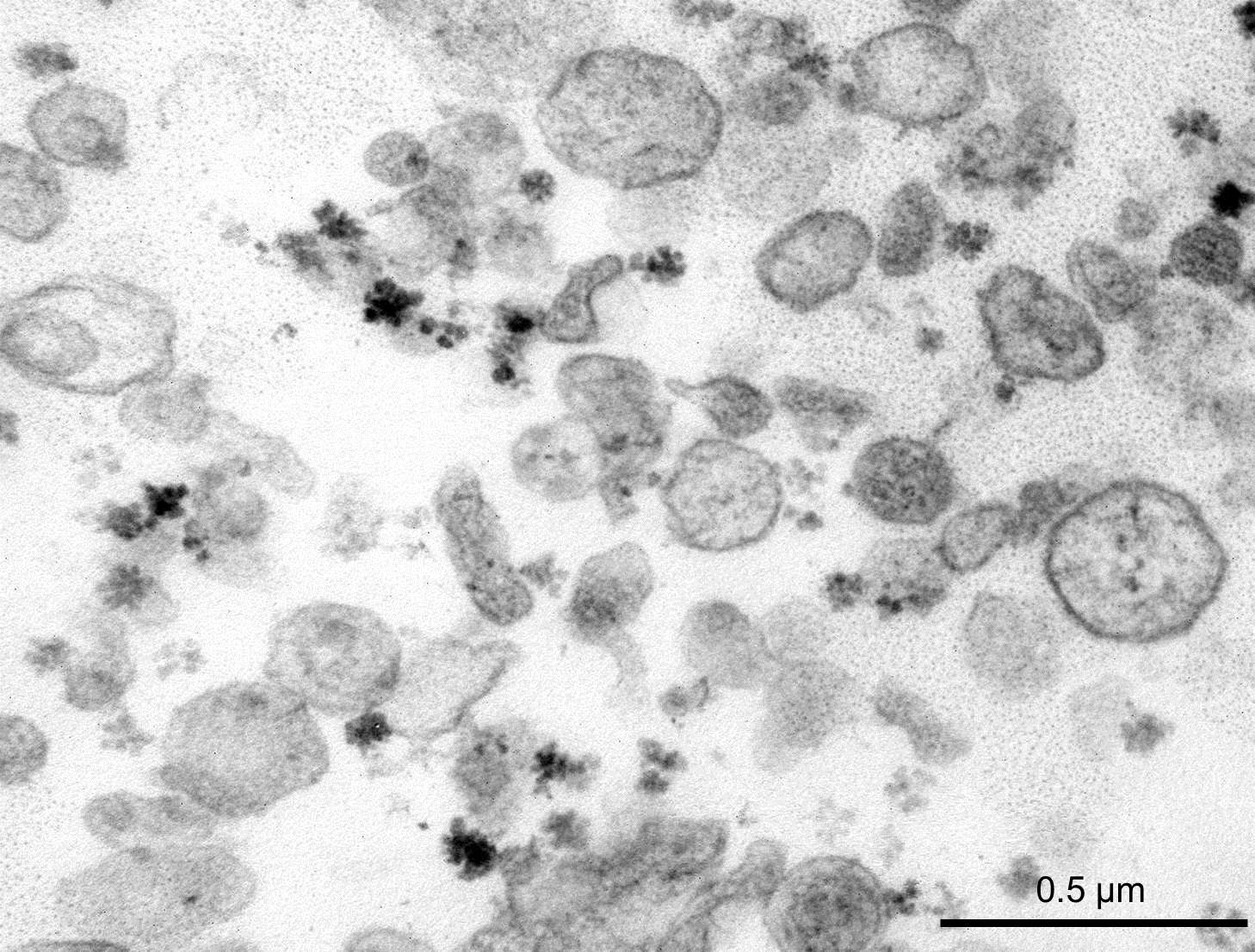
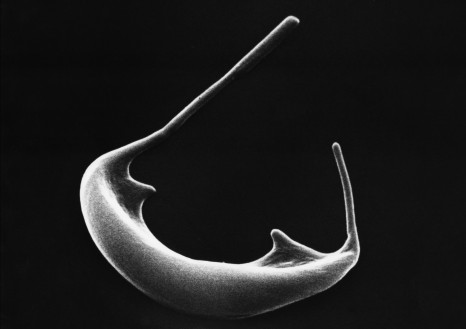
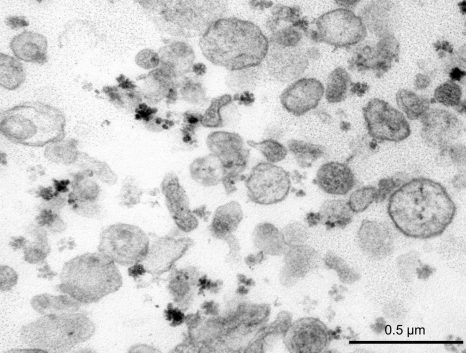
In 2012, a collaboration between a team Inserm Unit 970 (Mixed Research Unit 970 – Paris – Cardiovascular Research Center) and the Hepatology Service of Beaujon Hospital, AP-HP, showed that the microvesicles [1] in the blood of patients with cirrhosis contribute to vascular complications associated with this disease (Rautou, Gastroenterology 2012). A correlation between the original microvesicles hepatocyte rate in patients with cirrhosis and severity of liver disease was established.
From this observation, a prospective study funded by the National Agency for Research (ANR) was conducted among 242 patients with cirrhosis: 139 supported at the Beaujon Hospital, Clichy, AP-HP and 103 Barcelona, Spain.
The trial established was whether the microvesicles in the blood could predict the evolution of the patients.
The team measured and analyzed the microvesicles rate (annexin V, platelets, leukocytes, endothelial and hepatocellular) in plasma of these patients.
The results demonstrate that the extent of the original microvesicles rate hepatocyte greatly improves the prediction of 6-month mortality of patients with cirrhosis.
These biomarkers could provide a reliable tool to refine the prognosis of patients with cirrhosis to better predict their evolution and precisely select the most appropriate treatment for each individual.
The results also suggest that strategies to lower the microvesicles in the blood of patients with severe cirrhosis may be beneficial for patients.
[1] The microvesicles are extracellular vesicles. They are detectable in the plasma of healthy individuals as in that of the sick.