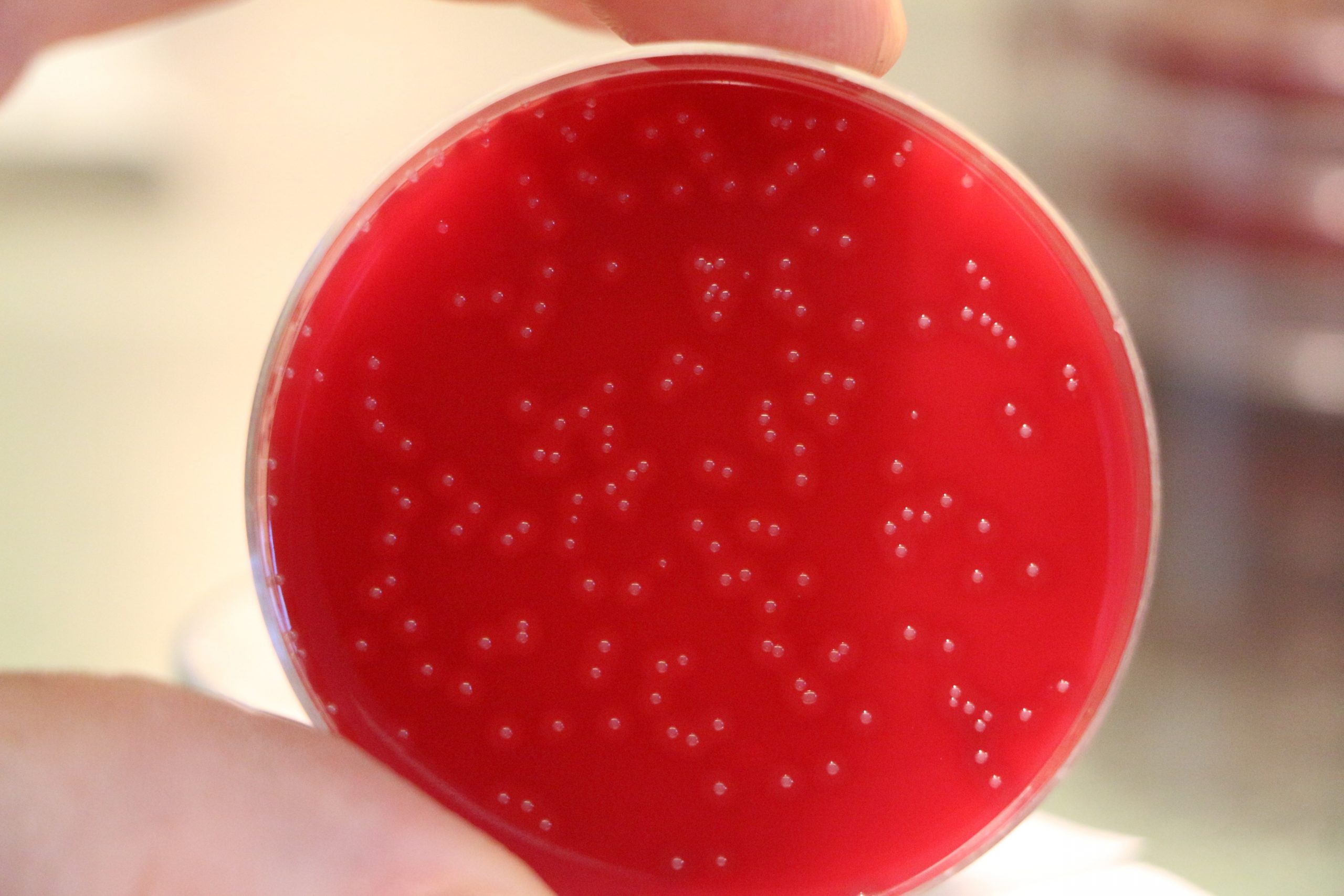
Colonies of Bordetella pertussis, the agent of whooping cough, on an agar plate. © Camille Locht/Inserm
Highly infectious and potentially life-threatening in infants, whooping cough, caused by the bacterium Bordetella pertussis, continues to circulate to a large extent throughout the world. Although the vaccines currently used protect against the onset of symptoms, they have limited durability and cannot prevent bacterial infection resulting in transmission between individuals. An international research team including Camille Locht, Inserm research director at the Center for Infection and Immunity of Lille (Inserm/Institut Pasteur de Lille/Université de Lille/Lille University Hospital/CNRS), has demonstrated, in a phase 2 clinical trial, the efficacy and safety of a nasal vaccine for whooping cough in adults. The results of their study, sponsored by ILiAD Biotechnologies and to be published in The Lancet, suggest that this new vaccine, BPZE1, which is potentially capable of preventing bacterial colonization of the respiratory tract, constitutes a valuable asset when it comes to breaking the epidemic chains of transmission of the disease.
Whooping cough is an infectious respiratory disease caused by the bacterium Bordetella pertussis. Highly contagious, it is known for causing fatal complications in infants.
Since the late 1990s, although the Tdap vaccine[1] has been used in industrialized countries mostly to fight whooping cough, the immunity it provides decreases over time, requiring the administration of boosters. In addition, although it helps to prevent the onset of symptoms, it does not prevent infection by the bacterium itself or its transmission to others. Therefore, whooping cough epidemics persist throughout the world, despite high rates of vaccination.
The development of a new whooping cough vaccine, called BPZE1, aims to make up for the shortcomings of Tdap in order to better fight these epidemics. A particularity of this “live attenuated” vaccine (containing an attenuated version of the bacterium) is that it is administered nasally, thereby mimicking the natural modes of transmission and colonization of Bordetella pertussis in the mucous membranes of the respiratory tract.
An international research team including Camille Locht, Inserm research director at the Center for Infection and Immunity of Lille (Inserm/Institut Pasteur de Lille/Université de Lille/Lille University Hospital/CNRS), in collaboration with the company ILiAD Biotechnologies, conducted a study to evaluate the efficacy and safety (non-toxicity) of BPZE1 in a phase 2 clinical trial with 300 healthy adult US participants.
In this study, the participants were divided into two groups: the first group received one nasal dose of BPZE1 and one intramuscular dose of placebo and the second group received one intramuscular injection of Tdap and one nasal dose of placebo. Three months later, half the participants from each of the two groups received one dose of BPZE1 (to simulate an attenuated natural infection), while the other half received intranasal placebo.
The research team found that, while the Tdap vaccine induced the secretion of high levels of Bordetella pertussis immunity markers in the blood, BPZE1 induced consistent immunity in both the nasal mucosa and the blood. In addition, within 28 days of the second nasal administration, 90% of the participants having initially received BPZE1 had no bacterial colonies in the nose. In the remaining 10%, colonization was low (fewer than 260 colonies per mL of mucus). In comparison, 70% of the patients vaccinated with Tdap had significant nasal bacterial colonization (nearly 14,325 colonies per mL).
Moreover, the research team did not see any serious adverse side effects from vaccination during the study.
Therefore, according to Locht, “the benefit/risk profile of BPZE1 is favorable: just one nasal administration induces safe and well-tolerated strong and long-lasting immunity, in both the blood and respiratory tract. And unlike Tdap, BPZE1 protects the mucous membranes from colonization by the bacterium”.
Indeed, given that Bordetella pertussis infects the respiratory tract and multiplies in its mucosa, immunity at this level could be essential in preventing epidemics of whooping cough.
“As this bacterium is highly infectious to humans, it is critical that a vaccine does not only target the prevention of disease but also the transmission of its causative bacterium and the speed at which the body eliminates it,” adds Locht, “which makes BPZE1 a relevant tool for preventing whooping cough infections and reducing the epidemic chains of transmission.”
Given that the participants in the aforementioned study were all adults over the age of 18, another study is ongoing to evaluate the efficacy and safety of BPZE1 in school-age children, as schools are a critical location for transmission of the disease.
More About the Development and Evaluation of BPZE1
In 2008, the European project CHILD-INNOVAC was launched under the auspices of Inserm in collaboration with 10 European partners, with the aim of developing an innovative nasal vaccine against whooping cough. This was the context for the development and patenting of the BPZE1 vaccine by a team from Inserm and Institut Pasteur de Lille led by Inserm research director and project coordinator, Camille Locht. In 2014, the research team published in PLOS ONE the first work evaluating the efficacy and safety of BPZE1 in a phase 1 clinical trial, following examination of the data by an Independent Data Monitoring Committee. An agreement was then reached between the Inserm Transfert platform, tasked with creating value from the intellectual property related to BPZE technology, and ILiAD Biotechnologies, in order to continue the development and evaluation of the vaccine. In 2020, a new phase 1 study conducted in collaboration with ILiAD Biotechnologies, and published in The Lancet Infectious Diseases, reinforced the clinical results from 2014 on the vaccine’s efficacy and safety.
[1] Tdap is an “acellular” vaccine, which does not contain whole bacteria but only certain proteins derived from Bordetella pertussis, which have the particularity of triggering a blood immune response. It combines vaccines against whooping cough, diphtheria, and tetanus, and is administered in France in three intramuscular doses to infants at 2, 4, and 11 months of age. Three boosters are recommended at around 16 months, 11 years and 26 years of age. Although better tolerated, it is more expensive and less effective than the whole cell pertussis vaccine (that contains the inactivated bacteria), which is still being used today in many low- and middle-income countries.






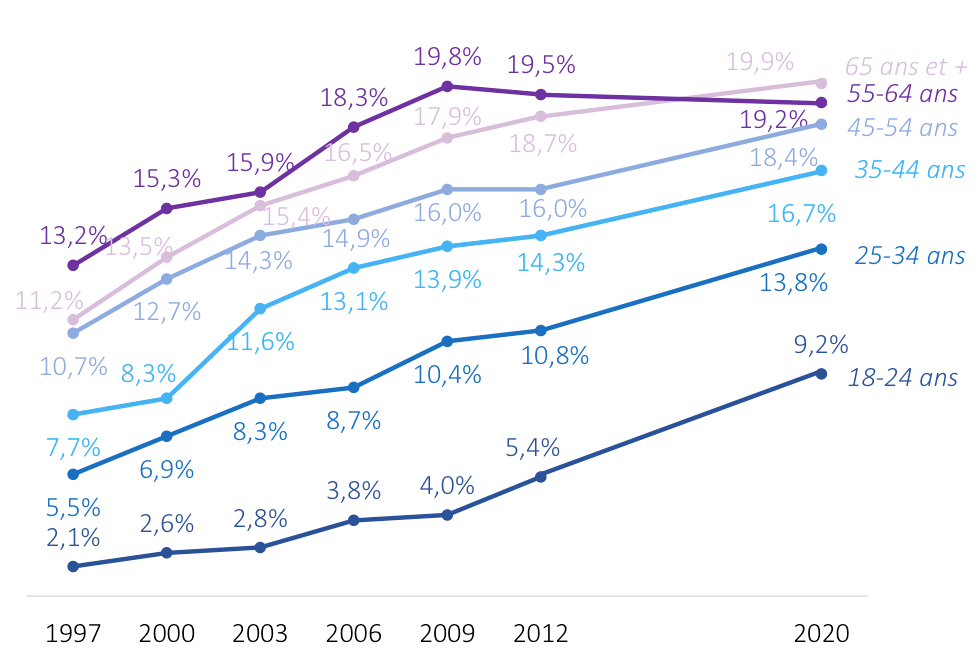 Evolution of the prevalence of obesity by age groups between the 1997-2012 Obépi-Roche surveys and the 2020 Obépi survey.
Evolution of the prevalence of obesity by age groups between the 1997-2012 Obépi-Roche surveys and the 2020 Obépi survey.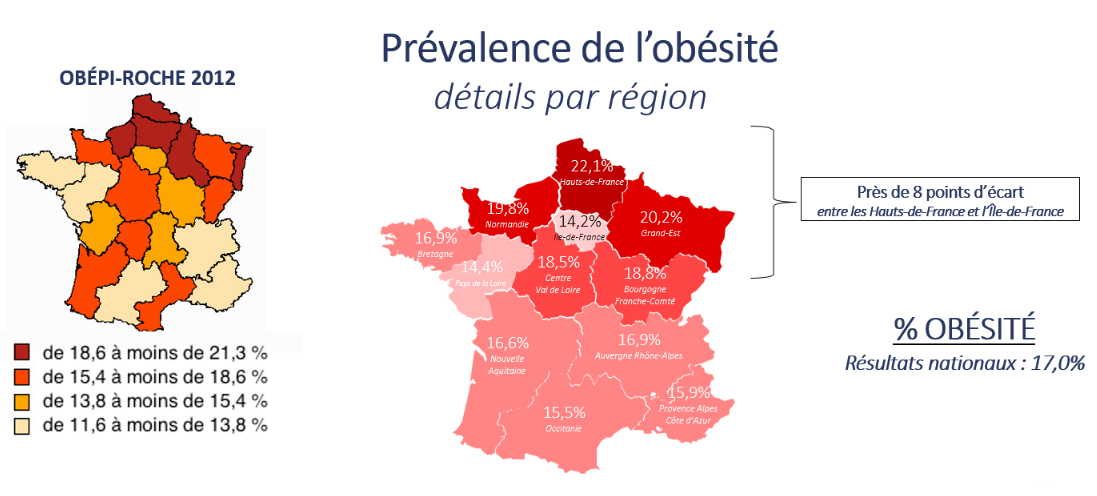 Geographic distribution of the prevalence of obesity in 2020 in the French regions
Geographic distribution of the prevalence of obesity in 2020 in the French regions