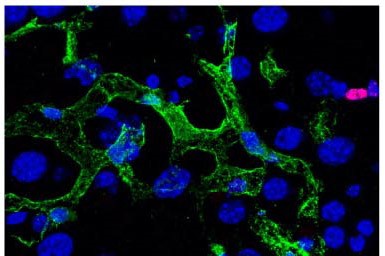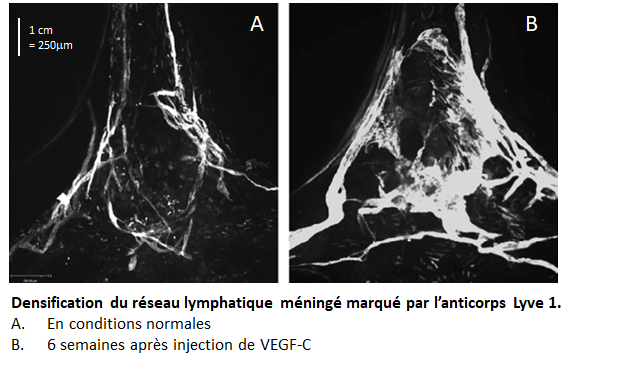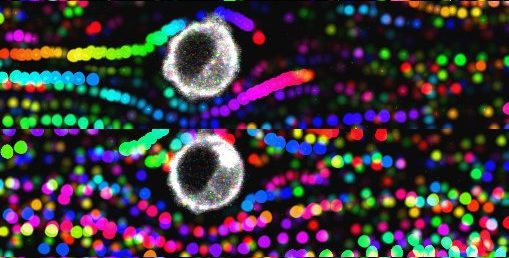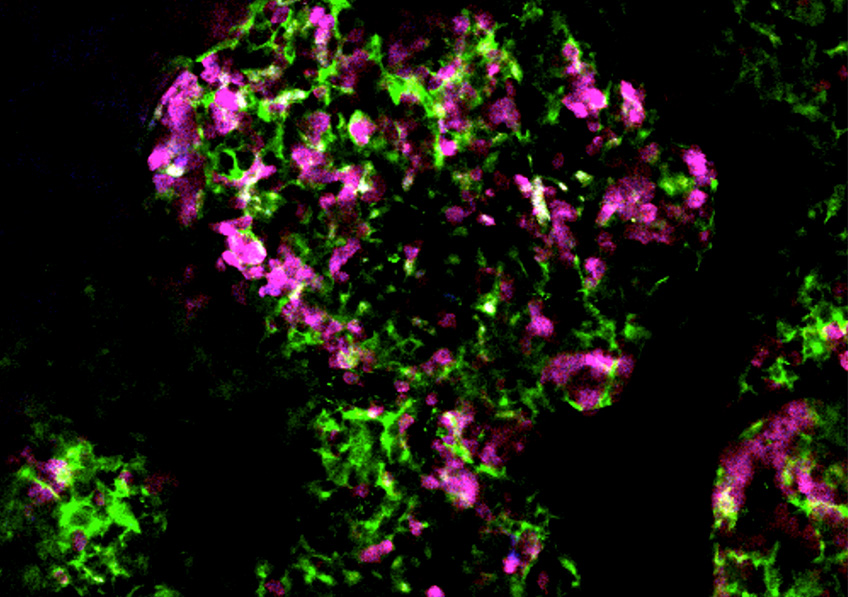
Image taken in vivo following the injection of anti-CD20 antibodies, showing cancer cells (in magenta) and macrophages (in green) attacking tumor cells. © Institut Pasteur – Dynamics of Immune Responses Unit
Monoclonal antibodies are part of the therapeutic arsenal for eliminating cancer cells. Some make use of the immune system to act and belong to a class of treatment called “immunotherapies.” But how do these antibodies function within the tumor? And how can we hope to improve their efficacy? Using innovative in vivo imaging approaches, scientists from the Institut Pasteur and Inserm visualized in real time how anti-CD20 antibodies, used to treat B-cell lymphoma, guide the immune system to attack tumor cells. Their findings were published in the journal Science Advances on February 19, 2021.
Anti-CD20 antibodies are used in clinical practice to treat patients with B-cell lymphoma, a type of blood cancer. The treatment, often used in combination with chemotherapy, has been largely proven to improve the prognosis of patients but some respond less effectively than others. It is therefore critical to better understand how these therapies actually work to then be able to overcome their weaknesses.
Scientists from the Dynamics of Immune Responses Unit (Institut Pasteur/Inserm), led by Philippe Bousso, visualized the effect of the anti-CD20 antibody within the tumor seconds following injection and relied on tumor cells that change color as they die. Using this strategy, they demonstrate that macrophages play an essential role in the efficacy of the therapy, by ingesting tumor cells coated with antibodies.
“What surprised us was the observation that this elimination phase, which begins immediately after the injection of the antibody, became less effective after just a few hours,” explains Capucine Grandjean, lead author of the study. The scientists also showed that the quantity of macrophages in the tumor was very likely to be insufficient to destroy all cancer cells.
In revealing some of the weaknesses of these antibodies, the scientists have opened up new avenues for the development of next-generation therapies. In particular, increasing the presence and activity of macrophages in the tumor represents a promising strategy to boost the efficacy of these therapeutic antibodies.