 MASH is a growing pandemic worldwide, with obesity and diabetes on the rise. It is also an area of significant unmet medical need. © François Pattou
MASH is a growing pandemic worldwide, with obesity and diabetes on the rise. It is also an area of significant unmet medical need. © François Pattou
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly referred to as nonalcoholic fatty liver disease (NAFLD), impacts roughly 30% of the global adult population. The disease spans from benign fat accumulation in the liver (steatosis) to its more severe form, metabolic dysfunction-associated steatohepatitis (MASH, formerly nonalcoholic steatohepatitis or NASH). MASH represents a dangerous progression, with the potential to cause cirrhosis, liver cancer, type 2 diabetes, and cardiovascular disease.
Despite its prevalence, MASH remains highly heterogeneous. Not all individuals follow the same clinical trajectory, and conventional treatment approaches often fail to account for these differences. Recognizing this gap, a groundbreaking study led by Prof. François Pattou and Prof. Stefano Romeo has redefined MASH by identifying two distinct subtypes with distinct risks and outcomes.
This transformative research, conducted at Lille University Hospital as part of the RHU PreciNASH project coordinated by Inserm, was made possible through collaboration with leading scientific teams from Inria, CNRS, the University of Lille, Lille University Hospital, and the Pasteur Institute of Lille, alongside international partners from Sweden, Italy, Belgium, and Finland. Published in Nature Medicine, the study marks a pivotal shift in the understanding and treatment of MASH.
Two Subtypes of MASH, Two Distinct Risk Profiles
The study identified and validated two distinct types of MASH based on histology and liver imaging, using data from European cohorts and the UK Biobank:
- Liver-Specific MASH: A genetically driven subtype with rapid progression of liver disease but a surprisingly low risk of cardiovascular complications.
- Cardiometabolic MASH: A high-risk profile linked to type 2 diabetes and cardiovascular diseases, alongside comparable liver disease progression.
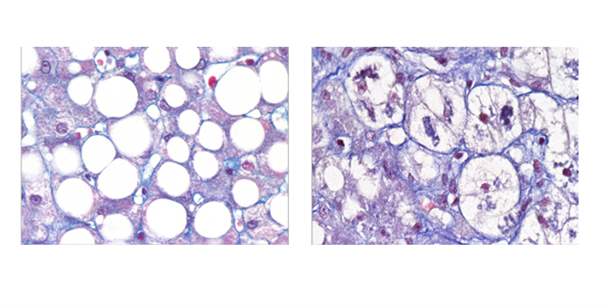
What makes this discovery groundbreaking is that both subtypes exhibit similar histological features under the microscope or on imaging, making them indistinguishable using traditional diagnostic methods. However, their markedly different clinical outcomes underscore the critical need for advanced diagnostic tools and personalized interventions.
Transforming Diagnosis and Treatment
This study empowers clinicians to move beyond one-size-fits-all approaches to treating MASH. By leveraging simple clinical markers—age, BMI, HbA1c, LDL cholesterol, triglycerides, and ALT—patients can be stratified into specific subtypes, enabling tailored treatments:
- Liver-Specific MASH: Focus on therapies to halt liver damage and prevent progression to cirrhosis or liver cancer.
- Cardiometabolic MASH: Emphasize aggressive management of metabolic and cardiovascular risks alongside liver disease treatment.
“This research marks a turning point,” says Prof. François Pattou. “We now have a clear path to develop subtype-specific treatments that can improve patient outcomes.”
Why This Discovery Matters
MASH is the most severe manifestation of MASLD, with the potential for devastating health consequences. However, its heterogeneity has often been overlooked, leading to inconsistent treatment outcomes.
“This manuscript offers a transformative perspective on MASH and its heterogeneous outcomes,” notes an anonymous reviewer. “Thoughtfully conducted on large, well-characterized cohorts, it opens new doors for precision medicine in this field.”
The Science Behind the Subtypes
The study utilized data from the French ABOS cohort of 1,389 individuals with obesity and validated its findings across three European MASLD cohorts (Italy, Belgium, and Finland), comprising 1,099 participants, as well as imaging data (MRI) from over 6,000 UK Biobank participants. By integrating clinical traits with liver transcriptomics and plasma metabolomics, researchers uncovered distinct biological pathways driving each subtype.
“This discovery sheds light on why current treatments often yield inconsistent results,” explains co-lead researcher Prof. Stefano Romeo. “It was a true ‘eureka’ moment for our team.”
A New Era for MASH Treatment
This breakthrough highlights the urgent need for subtype-specific care, paving the way for innovative treatments and personalized medicine. Future research will explore how these subtypes respond to lifestyle interventions, pharmacological therapies, and other treatments in diverse populations.
“We’ve always known MASH was heterogeneous,” concludes Prof. Romeo. “Now, we finally have a roadmap to turn these insights into real-world solutions for patients.”