©Brooke Lark on Unsplash
What if immune system efficacy against cancerous cells could be reinforced by a diet in which calories are not reduced but nutrients are precisely determined? This what Inserm researchers from Université Côte d’Azur, through a study of the effects of restrictive diets on tumor growth in mice, have been exploring. They have observed that a low-protein diet restricts tumor development by increasing immune response. The findings, to be published in Cell metabolism, have proved promising in understanding anti-tumor immunity in mice and pave the way for new studies in humans.
Despite the recent popularity of fasting in preventing cancer, in reinforcing chemotherapy and in extending life expectancy in patients with tumors, there is no solid scientific proof to support its efficacy at present. In reality, clinical trials are virtually non-existent in humans and the findings obtained from animal models are highly debatable. Prolonged calorie reduction can be an aggravating factor in the undernourishment and loss of muscle mass (sarcopenia) frequently associated with chemotherapy.
An Inserm team at Université Côte d’Azur decided to focus on a hypothesis by which modulating the intake of macronutrients (carbohydrates, fats and proteins) rather than that of calories, could restrict tumor growth. The researchers compared the effect of various diets, with varying levels of carbohydrates and proteins but the same number of calories, on tumor growth in mice. The results show that it was a low-protein and not a low-carbohydrate diet that had a positive impact on limiting tumor growth and prolonging life expectancy in mice.
Analysis of the tumor cell content of mice on a low-protein diet showed an increased quantity and more intense activity of the specific anti-tumor cells of the immune system. The researchers observed that the restriction of tumor growth was not due to inhibited cancer cell proliferation as could be believed, but to an increased efficacy of the immune response, also known as immunosurveillance, in destroying the cancerous cells.
When studying the molecular mechanisms linked to this phenomenon, the researchers observed that this strengthened immunosurveillance was linked to tumor cell secretion of immune system alert proteins, known as cytokines. According to the study, reducing proteins in the diet renders the available quantity of certain amino acids (constituents of proteins) insufficient – and these are substances to which cancer cells are highly sensitive. When access to amino acids is reduced, stress is triggered in the tumor cells, which then release cytokines and thereby activate a strong immune response against the tumor.



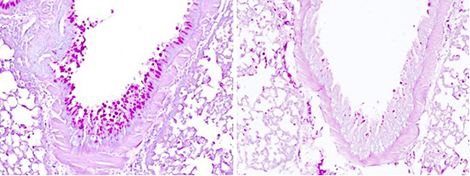
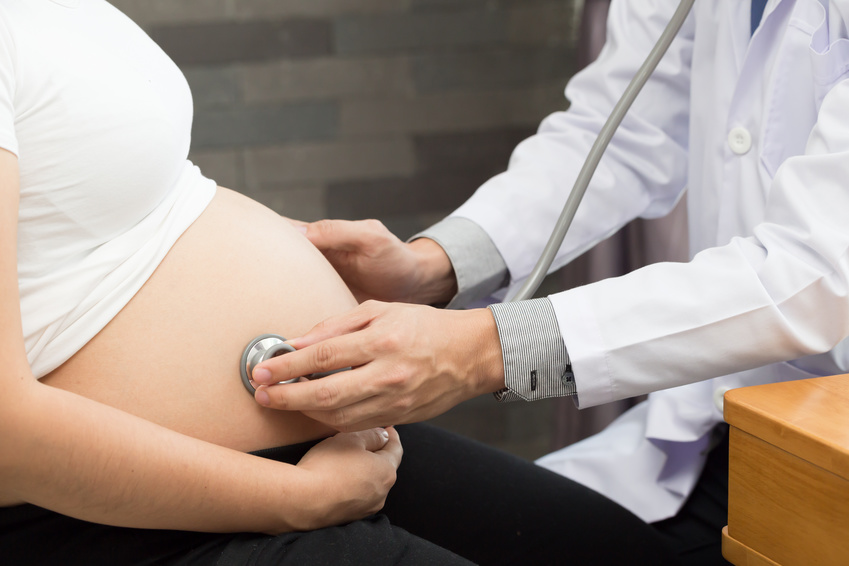
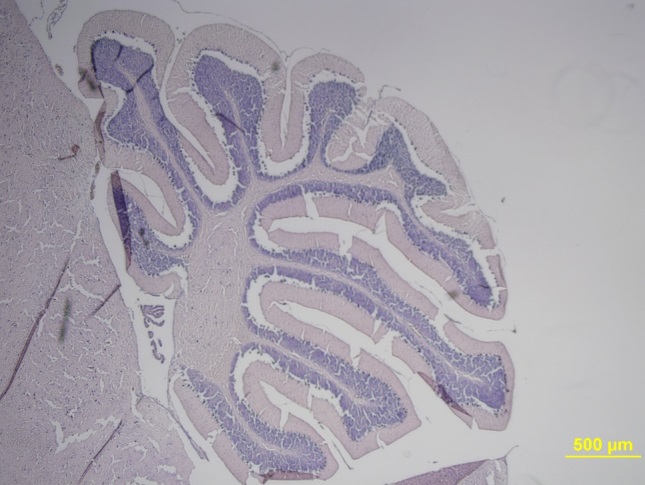
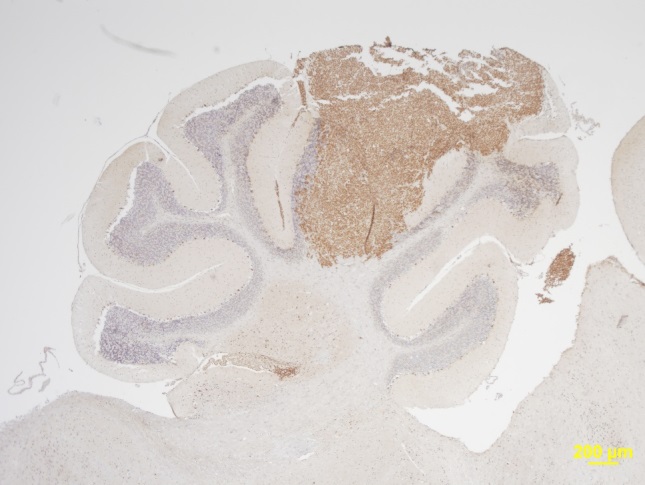



 ©Baranska et al., 2018
©Baranska et al., 2018