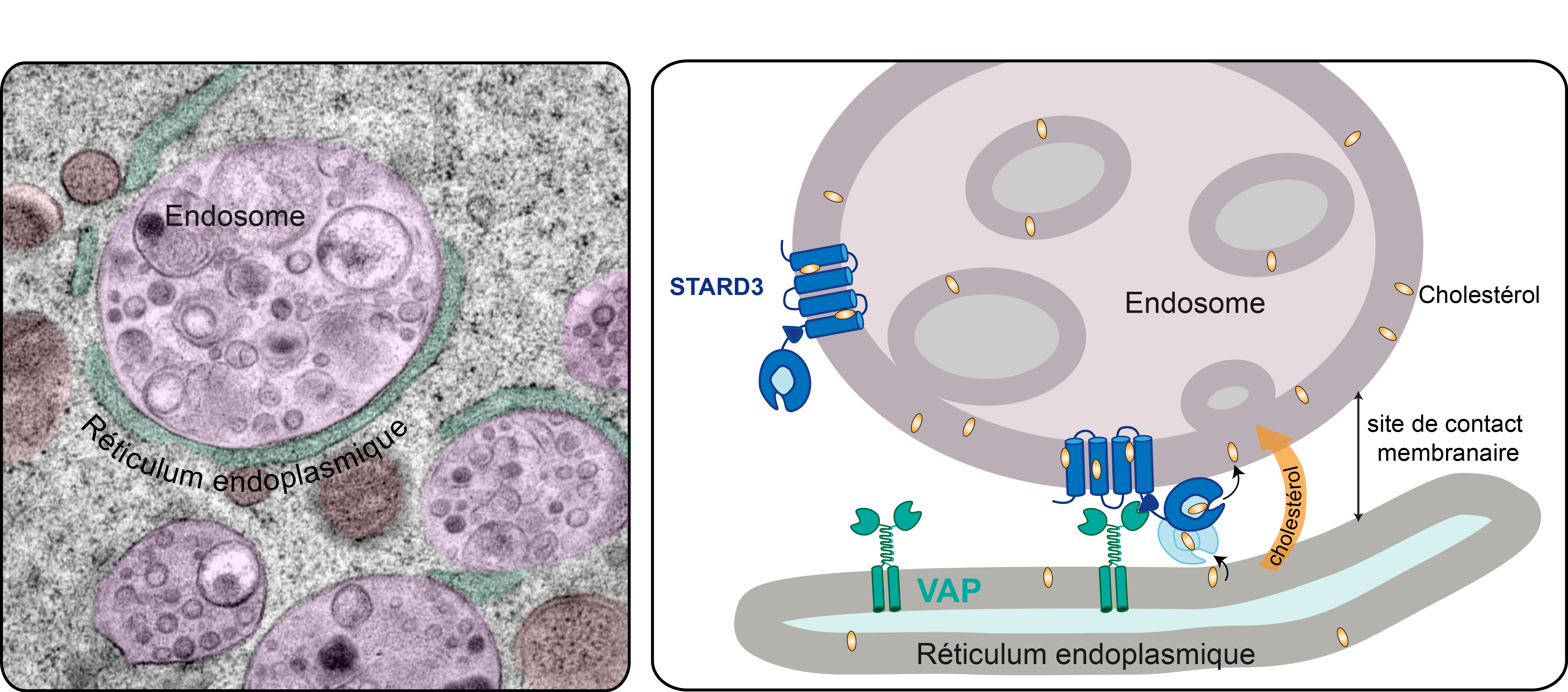
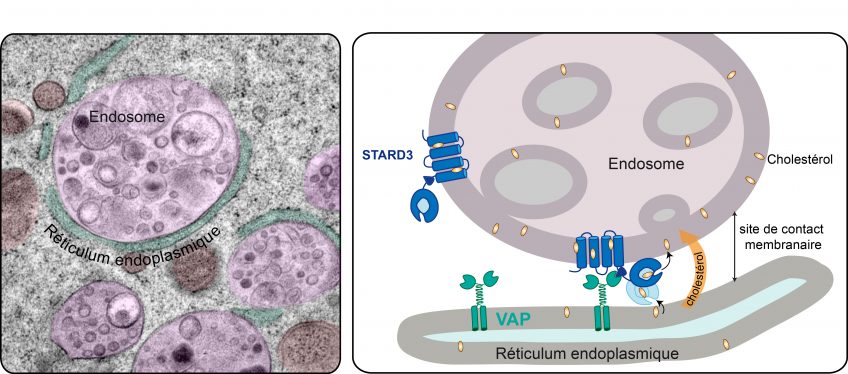
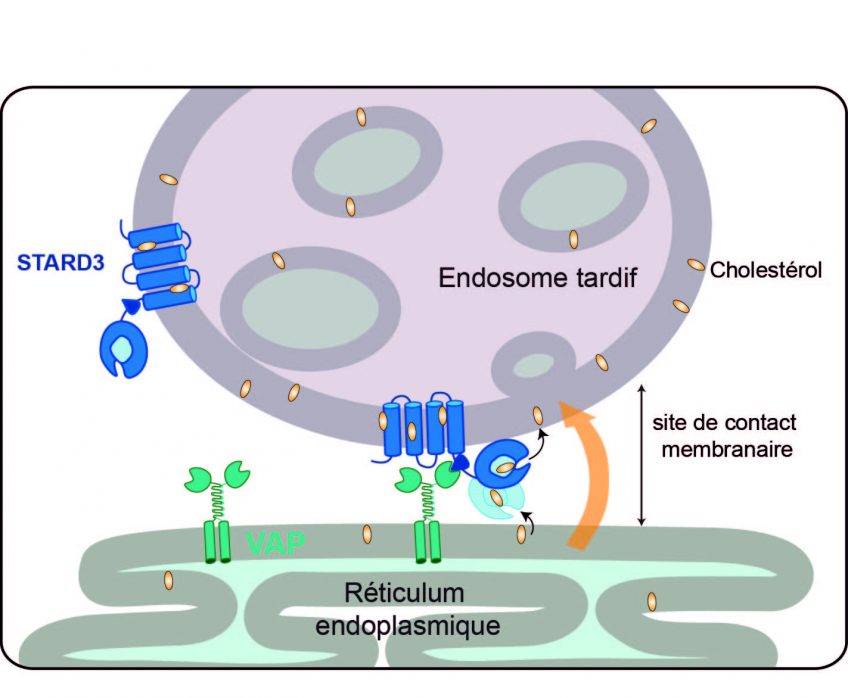
In a study published today in the journal EMBO Molecular Medicine1, the team led by Prof. Nicolas Lévy identifies the mechanism associated with the accumulation of progerin, a toxic protein produced in the course of ageing, and demonstrates the therapeutic potential of a new drug – MG132 – to treat progeria, a rare syndrome involving premature and accelerated ageing. Nicolas Lévy and his team have demonstrated the ability of this drug to considerably reduce progerin production and simultaneously degrade it. This drug, along with other compounds from the same family, is undergoing evaluation for the treatment of other rare diseases, as well as more common diseases including certain types of cancer.
This work, supported by Inserm, Aix-Marseille University, the A*Midex foundation and AFM-Téléthon, paves the way to a therapeutic trial and the development of compounds to reduce the effects of accelerated and physiological ageing.
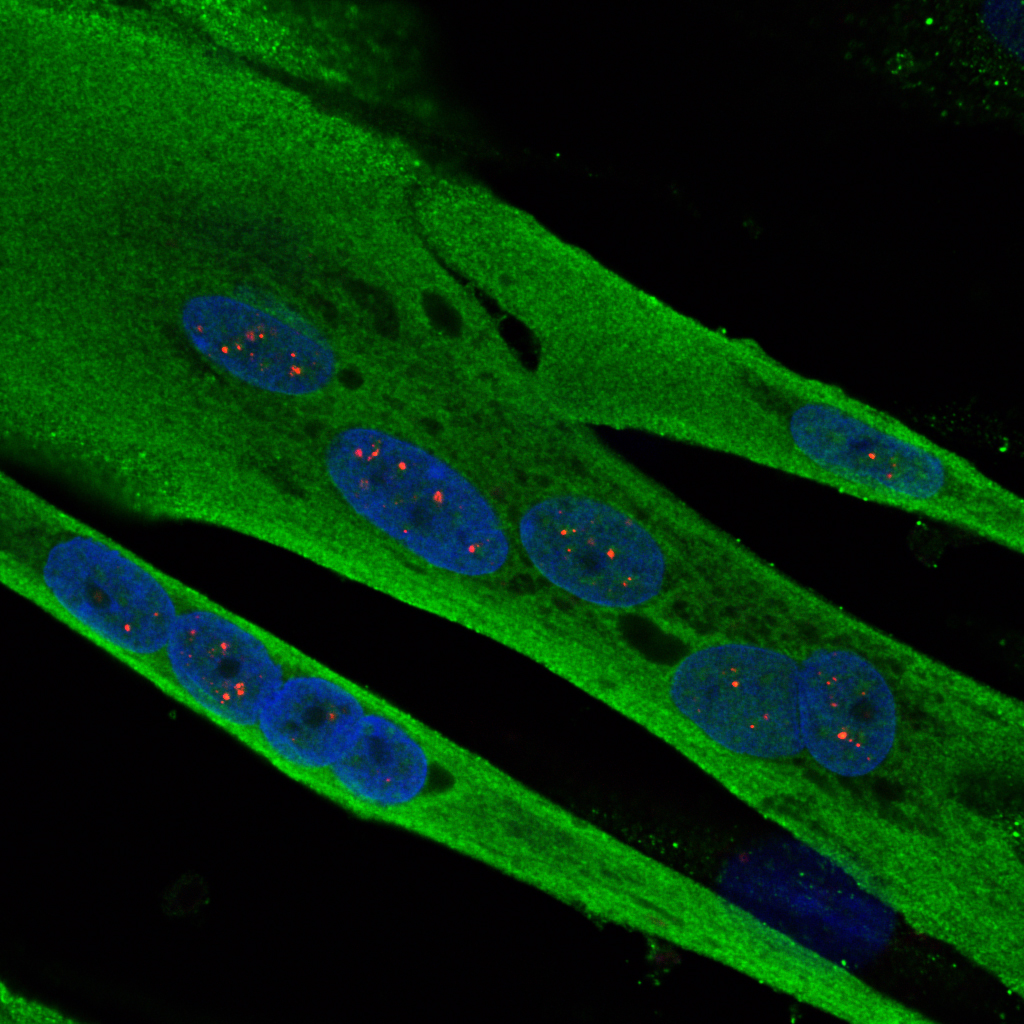
Hutchinson Gilford progeria syndrome (HGPS) is an extremely rare and severe genetic disease that causes precocious and accelerated ageing in children. Although it spares the brain functions, it progressively leads to ageing in the vast majority of the organs, with particularly dramatic consequences being observed in the skin, adipose tissue, cardiovascular system and bones. Constantly fatal, death usually occurs around the age of 13 years. This disease, which affects 1 birth per 10–20 million worldwide, is caused by a mutation in the LMNA gene taht leads to the production and accumulation of a toxic protein, progerin, in cells nuclei. Progerin causes serious cellular dysfunctions (defects in DNA breaks repair, failure of cell proliferation and differentiation, etc…). Progeria is thus a unique model for understanding major mechanisms involved in natural ageing. Since 2003, Nicolas Lévy and his team have identified the gene and mechanism inducing progeria and other premature ageing diseases, developed therapeutic approaches, and conducted the first European trial in 12 children affected with the disease.
In the study published today, Nicolas Lévy’s team – UMR_S910, Aix-Marseille University/Inserm – has identified the mechanism whereby progerin accumulates without being degraded, and has identified a family of drugs that not only allow a tremendous reduction in its initial production, but also the simultaneous elimination of the remaining produced progerin. This study, using cells from children affected with progeria as well as a mouse model developed within this same team£, paves the way for a clinical trial for progeria and other severe diseases of accelerated ageing. It will also be exploited in order to define the potential of each drug identified in the family, with respect to rare genetic diseases, cancers and natural ageing. For Dr Karim Harhouri, first author of the study, “These 5 years of work have enabled us to discover the real mechanism whereby progerin accumulates without being degraded, and a class of drugs that had not been exploited before, with a seemingly major therapeutic potential.”
“This work is part of the main thrust of our research in the area of rare genetic diseases, continuously aimed at translating knowledge of fundamental mechanisms into the most efficient possible treatments for our patients. This could not have been achieved without the convergence of talents, human skills and expertises to reach a common ambition, that of expanding effective treatments for our patients while reducing the access time; this is the philosophy we should be adopting, that of integrated research on care-related problems, and which we are upholding with the creation of the GIPTIS Institute*,” explains Nicolas Lévy, principal investigator, senior author of the study and proponent of the GIPTIS Institute*, which should open its doore in Marseille in 2020.
This work is the subject of a joint patent application – WO2016/113357 – holded by Aix- Marseille University, Inserm, AFM-Téléthon, CNRS and the ProGeLife** biotech company.
*GIPTIS : Genetics Institute for Patients, Therapies, Innovation and Science (www.giptis.com)